Mini Review
Plateau Waves, Intracranial Hypertension and Prognosis. How Identical Waves can Lead to Different Outcomes in Two Patients with Traumatic Brain Injury
Laura A. E. Cox MSc1, Bart P. Ramakers MD, PhD1 and Wilson F. Abdo MD, PhD1*
1Department of Intensive Care Medicine, Radboud University Medical Center, Nijmegen, The Netherlands
Corresponding author
Dr. Wilson F. Abdo, Department of Intensive Care Medicine, Radboud University Medical Center, Geert Grooteplein-Zuid 10, PO Box 9101, 6500 HB Nijmegen, The Netherlands, Tel: 00-31-24-36-17273; Fax: 00-31-24-3541612; E-mail: f.abdo@radboudumc.nl
Received Date: 04 Dec 2013
Accepted Date: 14 Jan 2014
Published Date: 17 Jan 2014
Citation
Cox LA, Ramakers BP, Abdo WF (2014) Plateau Waves, Intracranial Hypertension and Prognosis. How Identical Waves Can Lead to Different Outcomes in Two Patients with Traumatic Brain Injury. Enliven: J Anesthesiol Crit Care Med 1(1): 001.
Copyright
@ 2014 Dr. Wilson F. Abdo. This is an Open Access article published and distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
Elevated intracranial pressure (ICP) can lead to secondary brain damage and is associated with worst outcome in patients with traumatic brain injury. We present two clinical cases of high ICP due plateau waves. This, frequently occurring cause of high ICP, can cause confusion at the bedside if not recognized. High ICP due to plateau waves has a different prognostic implication and treatment algorithm compared to other causes of high ICP. We describe the pathophysiology and management of this phenomenon.
Keywords
Plateau waves; Intracranial pressure; Traumatic brain injury; Cerebral autoregulation; Outcome
Abstract
Elevated intracranial pressure (ICP) can lead to secondary brain damage and is associated with worst outcome in patients with traumatic brain injury. We present two clinical cases of high ICP due plateau waves. This, frequently occurring cause of high ICP, can cause confusion at the bedside if not recognized. High ICP due to plateau waves has a different prognostic implication and treatment algorithm compared to other causes of high ICP. We describe the pathophysiology and management of this phenomenon.
Introduction
In spite of the primary cause, elevated intracranial pressure (ICP) can lead to secondary brain injury and is associated with worst outcome [1]. Since the volume of the cranial cavity is fixed, an increase in one of its contents, e.g. due to edema or hydrocephalus, will lead to an increase of ICP. A frequently overlooked cause of recurrent episodes of elevated ICP is the phenomenon of Lundberg A waves, which are also called plateau waves. These are sudden elevations in ICP, with a plateau phase above 40mm Hg that lasts at least several minutes and with a subsequent sudden decrease in ICP to preexisting or lower values. Plateau waves are presumed to be triggered by a precipitating factor (e.g. decrease in blood pressure or saturation) leading to an intracerebral vasodilatory cascade and thus increased cerebral blood volume (CBV). The increase in CBV will result in higher ICP in case of a non-compliant intracranial system (low compensatory reserve, e.g. due to an already swollen brain). Normally, a rise in ICP above a certain threshold will result in a treatment to bring down the ICP value. The occurrence of high ICP spikes due to plateau waves can lead to confusion at the bedside due to two fundamental differences with other causes of ICP increase. First, although they are associated with very high ICP, plateau waves are not directly associated with worse outcome [2]. Secondly, management of plateau waves differs from regular ICP management. In contrast to plateau waves, regular ICP management will result in a treatment response when ICP exceeds 20mm Hg for several minutes.
Here we present two illustrative clinical cases with plateau waves, highlighting completely different outcomes due to the extent of the underlying pathology.
Clinical Case 1
A 32-year-old woman with no relevant previous medical history had a high speed scooter accident. At the scene of accident, she was unresponsive with some spontaneous movements of her right arm without pupil abnormalities. At our emergency department, CT-scanning showed fractures of the occipital condyle, vertebra C7 and vertebrae T3 and T4. Thoracoabdominal CT-scan revealed no other relevant injuries. The head CT-scan showed an acute right frontotemporal subdural hematoma of 8-9 mm thickness with a midline shift of 6 mm, several small cerebral hemorrhagic contusions, a frontal basilar skull and several facial fractures. There was no direct indication for surgical stabilization of the thoracic vertebral fractures. The cervical fracture was temporarily treated with a collar. The consultant neurosurgeon concluded that there was no indication to surgically evacuate the subdural hematoma. To monitor the ICP a right frontal intraparenchymal pressure transducer (Camino®, Integra neurosciences, Plainsboro, New Jersey, USA) was placed with an opening pressure of 18 mm Hg.
On the intensive care unit (ICU) aprotocol of a target ICP < 20mm Hg and a cerebral perfusion pressure (CPP) of > 60 mm Hg was followed. Despite escalating doses of sedation, she needed several bolus of 50cc of 10% saline to maintain ICP < 20mm Hg in the first 24 hours. A control head-CT showed a decreased subdural hematoma thickness and midline shift and small increases in the hemorrhagic contusions together with a minimal amount of right fronto-parietal subarachnoid blood. In the following days, hypertonic therapy was repetitively necessary to treat elevated ICP values.
.
After three days a different typical pattern with acute ICP spikes up to 80 mm Hg was seen. These patterns occurred at intervals of approximately 2 hours (as shown in Figure 1). A control head CT did not show any additional abnormalities (See Figure 2). These ICP spikes lead to a diagnostic problem as the on duty intensive care team, neurologist and neurosurgeon could not explain their occurrence and exact nature. ICP lowering therapy was intensified. An epileptic origin was ruled out as an EEG during ICP spikes did not show any epileptic discharges. After further consultation and literature review, the diagnosis of plateau waves was postulated and it was decided that plateau waves could be accepted to a maximum duration of 15 minutes (see discussion for this rationale). Most plateau waves were treated earlier, however. At the 11th day of hospitalization, weaning from sedation was started as ICP lowering measures had not been necessary for 24 hours. At the 16th day of admission an anterior cervical spondylodesis of C6-C7 was performed. At day 18 she was extubated and was awake and following commands. One month after admission she was transported to another hospital for further rehabilitation and had a good recovery.
Clinical Case 2
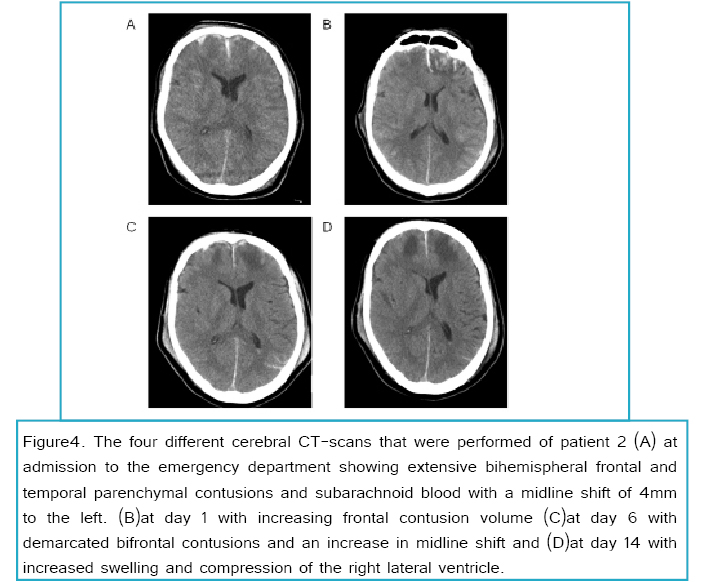
A 58-year-old man was transferred to our emergency department because of a traumatic head injury due to a scooter accident. Besides a cholecystectomy and gout he had no other previous medical history. His Glasgow Coma Scale at the trauma scene was E1M5V3, with normal pupil reactivity and no signs of lateralization. The head-CT showed a small right frontal epidural hematoma, extensive bihemispheral frontal and temporal parenchymal contusions and subarachnoid blood with a midline shift of 4mm to the left with radiological signs of a possible transtentorial herniation. Also fractures of the right frontal skull and left mastoid were noticed. Further trauma work-up did not show any relevant abnormalities.
He was transferred to the ICU where a right frontal intra parenchymal pressure transducer was placed (Camino®, Integra neurosciences, Plainsboro, New Jersey, USA), with an opening pressure of 20 mm Hg. Due to the necessity of ICP lowering therapies (sedation and hypertonic saline), a control head-CT was made 9 hours after admission. The head-CT showed an increased volume of the cerebral contusions. However, there was more space in the basal cisterns. The consultant neurosurgeon found no indication for decompressive cranieactomy or other neurosurgical intervention. Due to the necessity of deep sedation and repetitive hypertonic treatment, the ICP lowering treatment was intensified and hypothermia was induced with a target body temperature of 32-34°C. This resulted in lower ICP values.
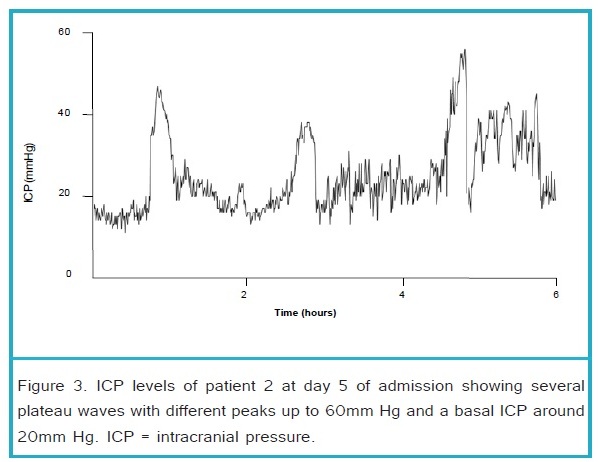
.
At day 3 of hypothermia, slow re warming of body temperature with a rate of 2°C/day was started. At that time he had an ICP < 20mm Hg without any rescue therapies for 24 hours. The next day, when the patient was 36°C, sedation was slowly decreased. However, ICP increased the same day to levels > 25 mm Hg. Hypothermia was intensified and sedation was restored to previous dosages. ICP recordings showed two patterns; a) basal ICP level difficult to maintain below 20mm Hg, b) additional ICP spikes to values > 40 mm Hg lasting up to 30 minutes. These ICP spikes were frequently aborted by hypertonic saline, or increasing vasopressor infusion to maintain an adequate CPP (see Figure 3). A control head CT showed increased cerebral swelling and a midline shift of 6mm. The basal ICP level gradually increased in the following days with frequent plateau waves. At day 15 a control head-CT (shown in Figure 4) showed further deterioration with an increased swelling and obliteration of the basal cistern. It was concluded that he had a dismal prognosis due to the extensive abnormalities on the head-CT, long lasting and frequent high ICP still necessitating extensive ICP lowering therapy at day 15. It was decided that the ICP transducer would be removed and sedation would be decreased in decrements of 50% per day to allow for formal neurological examination. 12 hours after the first reduction of sedation his pupils became dilated and unresponsive to light with additional loss of other brain stem reflexes. Patient died the following day.
Discussion
Plateau waves were first described in 1950 and were coined as ‘A’ waves by Lundberg in the 1960 [2]. These sudden increases in ICP above 40 mm Hg with concomitant decreases in CPP last longer than 5 minutes. During a plateau wave, ICP increases in a period of a few minutes to values sometimes as high as 100 mm Hg. In most instances arterial blood pressure (ABP) remains relatively stable, which leads to markedly decreased CPP (CPP = mean ABP – ICP). Figure 5 shows the ICP, CPP, heart rate and mean ABP of our patients during a plateau wave. Plateau waves terminate in a rather abrupt manner spontaneously or in response to treatment. Plateau waves occur in up to 20% of patients suffering from various cerebral pathological conditions such as severe traumatic head injury [2,3]. Although they occur frequently, it is our experience that they are often overlooked and lead to confusion at the bedside. Mostly other and better known causes of high ICP are considered and investigated (see table 1), and waveform analysis does not occur frequently. This is in part because ICP spikes are frequently treated aggressively irrespective of their physiology. As a consequence, plateau waves as origin for high ICP are frequently unnoticed. Only when recurrent ICP spikes wax and wane spontaneously without treatment, the nature of plateau waves is noted more easily.
| Differential diagnosis of acute elevations in ICP | Diagnostic modality |
|
Edema following traumatic brain injury |
Brain imaging |
|
Subdural/epidural/subarachnoidal/intraparenchymal hemorrhage |
Brain imaging |
|
Venous sinus thrombosis |
Venography using brain imaging |
|
Secondary ischemia |
Brain imaging, PbtO2 |
|
Central nervous system infections |
CSF analysis, brain imaging |
|
Plateau waves |
ICP Waveform analysis |
|
Hydrocephalus |
Brain imaging |
|
Seizures |
EEG |
Brain imaging = CT or MRI. CSF = Cerebral spinal fluid.PbtO2 = brain tissue oxygen tension
Table 1. Differential diagnosis of acute elevations in ICP in patients with traumatic brain injury.
Pathophysiology
The presumed pathophysiology behind this phenomenon is the presence of an intact cerebral vasomotor reactivity (autoregulation) in combination with a non-compliant low compensatory intracranial content. Maximal cerebral vasodilatation can occur during a plateau wave as has been shown by other markers of cerebral autoregulation [4]. Intracerebral vasodilatation results in an increased cerebral blood volume leading to a rise in ICP and a concomitant decrease in CPP. Cerebral vasodilatation can be provoked by several different situations, such ashypoxia, hypercapnia, hypotension or use of vasodilatory drugs [2,3,5]. Although CPP is decreased during a plateau wave, the existence nor the frequency or height of the peak ICP during plateau waves are associated with worse outcome [2]. Nonetheless, plateau waves that exist for more than 30-40 minutes do have a negative effect on outcome as prolonged periods of low CPP can lead to cerebral ischemia [2]. Patients with plateau waves have a significantly lower mortality rate compared to patients without plateau waves [3,5]. This seems counter intuitive. However, plateau waves are induced by a cerebral vasodilatory cascade to counteract a trigger that could disrupt oxygen delivery to the brain, e.g. hypoxia or hypotension. Thus, for plateau waves to occur intact cerebral auto regulation should be present and this may be related to explain the better reported outcomes in patients with plateau waves.
Treatment
Plateau waves either resolve spontaneously or in response to ICP lowering treatment, e.g. hypertonic saline, a cerebral vasoconstricting agent or short trail of hyperventilation [2,3]. A very short trail of hyperventilation to abort plateau waves can be used if necessary [3]. However, the prophylactic use of (longer trails of) hyperventilation is strongly discouraged as it results in cerebral vasoconstriction with decreased cerebral oxygenation and risk of iatrogenic cerebral ischemia [6,7]. Indomethacin, a potent cerebral arteriolar vasoconstrictor, has shown to reverse the vasodilatory cascade during a plateau wave and improve cerebral oxygenation [8]. One should always be aware of possible vasoconstriction induced negative effects on cerebral perfusion and oxygenation. Use of potent cerebral vasoconstrictors should preferably be used in the presence of multimodality monitoring such as brain oximetry or microdialysis in order to track possible side effects of such a treatment in the individual patient [9,10].
In our first clinical case, basal ICP was mostly maintained below < 20mm Hg despite of the frequent necessity of ICP lowering therapy. After 3 days, she developed a period of 9 days with frequent plateau waves. Our second case also developed recurrent plateau waves. However, long lasting refractory high ICP, not due to plateau waves, was also present. The total time of ICP above the desired threshold was much larger in our second case. This ‘ICP dose’ has been shown to be associated with outcome [11]. Additionally, in our second case progressive extensive intracranial abnormalities were present leading to brain herniation and dead. The extent of CT abnormalities is strongly associated with outcome [12]. We cannot exclude that the frequent high plateau waves contributed to the progressive secondary injury in our second case.
Conclusion
Plateau waves occur frequently and can be overlooked in patients on the intensive care unit. They occur when cerebral auto regulation is largely intact. Plateau waves are not necessarily associated with worse outcome. Therefore, a different ICP treatment approach is recommended to avoid iatrogenic complications due to aggressive ICP treatment.
Both clinical cases showed identical plateau waves but different outcomes. Although, our second case had a better GCS at the trauma scene, the CT abnormalities, the ‘ICP dose’, and higher age may have contributed to worse outcome.