Research Article
Peri-implant Alveolar Bone Augmentation Using Allogeneic Marrow-Derived Stem Cells; A Pilot Study in the Canine Mandible
Luisa F. Echeto1, Ingeborg J. De Kok2, Debra Sacco3, Susan J. Drapeau4, and Lyndon F. Cooper5*
1Clinical Associate Professor and Director, Division of Prosthodontics and Pre-doctoral Prosthodontics Program, University of Florida, College of Dentistry, Gainesville, FL
2Associate Professor, Department of Prosthodontics, and Bone Biology and Implant Therapy Laboratory, University of North Carolina at Chapel Hill, Chapel Hill, NC
3Private Practice, Oral and Maxillofacial Surgery Associates, Chapel Hill, NC
4Director, Biologics R&D, Medtronic, Minneapolis, MN
5Stallings Distinguished Professor, Department of Prosthodontics and Director, Bone Biology and Implant Therapy Laboratory, University of North Carolina at Chapel Hill
Corresponding author
Lyndon F. Cooper, Distinguished Professor, Department of Prosthodontics, School of Dentistry, University of North Carolina, 330 Brauer Hall, CB# 7450, Chapel Hill, NC 27599-7450, Tel: (919) 537-3437; Fax: (919) 537-3977; E-mail: Lyndon_Cooper@unc.edu
Received Date: 01st May 2014
Accepted Date: 23rd December 2014
Published Date: 26th December 2014
Citation
Echeto LF, De Kok IJ, Sacco D, Drapeau SJ, Cooper LF (2014) Peri-implant Alveolar Bone Augmentation Using Allogeneic Marrow-Derived Stem Cells; A Pilot Study in the Canine Mandible. Enliven: J Genet Mol Cell Biol 1(4): 003.
Copyright
@ 2014 Dr. Lyndon F. Cooper. This is an Open Access article published and distributed under the terms of the Creative Commons Attribution License, that permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
Vertical bone augmentation at endosseous implants is a technically challenging procedure that, when successful, can contribute to the biomechanical and esthetic value of implant-supported dental rehabilitations. One approach is the creation of a protected space for host cell ingrowth and subsequent bone formation. The aim of this pilot study performed in the beagle dog model was to examine the feasibility of an alternative approach for concurrent implant placement and vertical bone augmentation using an allogeneic marrow-derived stem cell-based construct without a barrier membrane. Allogeneic marrow-derived stem cells were obtained from iliac crest aspirations from donor animals and subsequent cell culture expansion. Alveolar defects (7 mm long x 4 mm deep) were created six weeks following tooth extraction. Central to the defects, 3.5 x 8 mm endosseous implants were placed at a depth of 4.5 mm. The 3.5 mm of exposed implant was either a) left exposed to the forming blood clot, b) covered with a HA/TCP matrix, c) covered with an allogeneic-stem cell-loaded matrix or d) covered with an autogenous bone graft. After 6 weeks, block sections of the mandible were prepared for histological evaluation of healing. For each implant (2 per test group), three sections were made parallel with the long axis of the implants. The histological appearance of bone was scored for each dental implant at both the implant-host bone interface and the implant-regenerated bone interface. The results demonstrated that allogeneic stem cell-loaded devices supported the formation of a bone-to-implant interface along the entire vertical augmentation surface of the implants and along the bone-to-graft interface. Vertical augmentation was also achieved using autogenous bone. However, in the absence of allogeneic stem cells or in the absence of any matrix graft, bone regeneration failed to occur. This initial success with vertical alveolar bone augmentation at dental implants using an MSC-based tissue engineering approach suggests a number of avenues to improve or simplify current regenerative therapies in dentistry.
Keywords:
Mesenchymal stem cell; Tissue engineering; Alveolar bone regeneration; Dental implant
Abstract
Vertical bone augmentation at endosseous implants is a technically challenging procedure that, when successful, can contribute to the biomechanical and
esthetic value of implant-supported dental rehabilitations. One approach is the creation of a protected space for host cell ingrowth and subsequent bone
formation. The aim of this pilot study performed in the beagle dog model was to examine the feasibility of an alternative approach for concurrent implant
placement and vertical bone augmentation using an allogeneic marrow-derived stem cell-based construct without a barrier membrane. Allogeneic
marrow-derived stem cells were obtained from iliac crest aspirations from donor animals and subsequent cell culture expansion. Alveolar defects (7
mm long x 4 mm deep) were created six weeks following tooth extraction. Central to the defects, 3.5 x 8 mm endosseous implants were placed at a
depth of 4.5 mm. The 3.5 mm of exposed implant was either a) left exposed to the forming blood clot, b) covered with a HA/TCP matrix, c) covered
with an allogeneic-stem cell-loaded matrix or d) covered with an autogenous bone graft. After 6 weeks, block sections of the mandible were prepared
for histological evaluation of healing. For each implant (2 per test group), three sections were made parallel with the long axis of the implants. The
histological appearance of bone was scored for each dental implant at both the implant-host bone interface and the implant-regenerated bone interface.
The results demonstrated that allogeneic stem cell-loaded devices supported the formation of a bone-to-implant interface along the entire vertical
augmentation surface of the implants and along the bone-to-graft interface. Vertical augmentation was also achieved using autogenous bone. However,
in the absence of allogeneic stem cells or in the absence of any matrix graft, bone regeneration failed to occur. This initial success with vertical alveolar
bone augmentation at dental implants using an MSC-based tissue engineering approach suggests a number of avenues to improve or simplify current
regenerative therapies in dentistry.
Introduction
The development of guided bone regeneration for dental implant procedures has expanded the opportunities for endosseous implant placement [1-4]. This approach dictates that a space should be created extra-skeletally and protected by a barrier membrane to stabilize the blood clot and to exclude non-osteogenic cells [5-6]. Within this space, the slowly migrating osteogenic cells that come from the underlying marrow form new bone. Additional requirements include prevention of acute inflammation and mechanical stability of the wound [7]. This barrier membrane approach to Guided Tissue Regeneration has been extended to formation of bone around titanium dental implants [8]. However, limitations to the use of barrier membranes alone exist, such as a lack of osteogenic cells within the vicinity of the defect [9].
Pragmatic limitations are revealed in challenging vertical alveolar bone augmentation. Previous investigations reveal limitations in different clinical methods for obtaining clinically significant alveolar bone augmentation [10]. In a systematic review focusing on vertical bone augmentation, the authors considered GBR, distraction osteogenesis and onlay bone grafting. Vertical bone gain was reported to be 2–8 mm, but relatively high percentage of treatment complications was revealed. A general conclusion was that the generalizability of the approach is limited at this time [11]. In an earlier review, Aghaloo and Moy (2007) [12] indicated that existing evidence suggested that implant survival may be a function of the residual bone rather than grafted bone. Continued research is needed for clinical improvement [13].
The advances in bone-tissue engineering using marrow-derived mesenchymal stem cells (MSCs) offers the clinical opportunity to directly place appropriate numbers of osteogenic cells in desired extra-skeletal spaces to direct bone formation [14]. MSCs are rare cells resident among the bone marrow that can be selectively isolated from an aspirate and expanded several million-fold to generate tissue engineering devices containing relatively high numbers of cells [15]. Based upon the matrix environment, the MSC can be intentionally differentiated in vitro to adipose, tendon, muscle, cartilage or bone [16]. These developments in cell biology have led to pre-clinical evaluations of potential uses for diverse orthopedic indications [17-19]. An additional focus of MSC-based regenerative therapy is in the dental arena.
Vertical alveolar bone augmentation is a challenging clinical scenario of significant importance in dentistry. The simplification and expansion of this therapy might involve the direct circumferential application of a solid or semi-solid matrix at the exposed implant without need for an exclusionary membrane. To direct bone formation, an osteoinductive stimulus (e.g. BMPs) or an osteogenic precursor (e.g. MSCs) may be included. It was the aim of this introductory study in the beagle dog model to determine whether or not allogeneic (donor-derived) MSCs adherent to a hydroxyapatite/tricalcium phosphate (HA/TCP) matrix could direct vertical bone augmentation concurrent with placement of a cpTitanium endosseous implant without the application of an exclusionary membrane.
Materials and Methods
This pilot study using the beagle dog model was designed as a randomized, controlled investigation with four treatment arms; MSC allograft plus HA/TCP matrix, HA/TCP matrix only, autogenous bone, or no graft treatment. The research proposal was approved by the Institutional Animal Care & Use Committee (IACUC), at the University of North Carolina. All surgical procedures were performed under general anesthetic according to the guidelines of the IACUC and the Department of Laboratory Animal Medicine.
MSC harvest and expansion – Bone marrow aspirations were drawn from animals used in another study. The marrow donor animals were from a colony different from the recipient animals; this method has been shown in parallel studies to give a high probability of mismatched donor-recipient pairs, employed in the allogeneic construct group [20].
Bone marrow aspirations from the posterior ilium were performed using standard procedures with a biopsy needle. Nine milliliters of marrow were drawn into a 10 cc syringe containing 1000U heparin and transferred to the laboratory for MSC isolation and expansion. Marrow samples were washed with saline, followed by centrifugation over a 1.073 g/ml Percoll density cushion. The interface layer was removed and the cells were washed in phosphate buffered saline solution, counted and were plated in tissue culture flasks in Dulbecco’s Modified Eagle’s Medium (DMEM) containing 10% fetal bovine serum (FBS). Non-adherent cells were washed from the culture during twice weekly feedings [17]. When the plated MSCs were near confluence (3-4 weeks), they were trypsinized, passaged, and cryopreserved for use in alveolar bone grafting experiments.
Bone tissue engineering construct preparation - HA/TCP (60%/40%) was obtained in a porous block form (Biomatlante,Vigneux de Bretagne, France). Twelve (12) cylinders, 5.5 mm in diameter and 3 mm thick with a 3.5 mm central hole, were prepared. The machined matrices were then sonicated to remove particulate material and sterilized by heating at 250°C for 4 hours. Allogeneic MSCs (passage 1 following cryopreservation) were suspended at a concentration of 10 x 106 cells/ml in DMEM (without serum) and were applied to six of the prepared cylinders in a closed, sterile system using negative pressure to assure loading within the matrix pores. The constructs remained in the MSC suspension for 48 hours. To remove non-adherent cells, the constructs were dispensed into 10 ml of sterile saline solution just prior to engraftment.
Bone autograft preparation – A 5.5 mm diameter core of autogenous cortical bone was excised from the buccal plate of the mandible lateral to the first molar roots. Central to this core, a 3.5 mm diameter osteotomy was prepared to create 6grafts for placement circumferentially around the dental implant. The graft was immediately placed at the selected dental implants.
Animal surgery - The P2 and P4 bilateral mandibular teeth were extracted in six (6) beagle dogs. Six weeks after extraction, saddle defects (3 mm deep x 7 mm long) were created in the P2 and P4 regions of the mandible via a mid-crestal incision (Figure 1a, 1b). 4.5 mm deep osteotomies were prepared to 3.35 mm diameter for implant placement using sequential drilling and copious irrigation. Twenty four (24) 3.5 mm diameter x 8 mm long implants (Microthread; AstraTech AB, Waltham, MA) were placed to the 4.5 mm depth leaving 3.5 mm of the implant exposed above the alveolar defect (Figure 1c). The 3.5 mm of exposed implant was either a) left exposed to the forming blood clot, b) covered with an HA/TCP matrix, c) covered with an allogeneic-stem cell-loaded HA/TCP matrix or d) covered with an autogenous bone graft. Six (6) cylindrical constructs or autogenous bone grafts were placed directly onto the implant (Figure 1d) and (Table 1).
| Dog 1 | Dog 2 | Dog 3 | Dog 4 | Dog 5 | Dog 6 | |||||||
| P4 | P2 | P4 | P2 | P4 | P2 | P4 | P2 | P4 | P2 | P4 | P2 | |
| Matrix | X | X | ||||||||||
| Matrix+Cell | X | X | X | X | ||||||||
| Auto Bone | X | X | X | X | ||||||||
| No Graft | X | X | ||||||||||
The twenty-four (24) surgical sites were then closed using vertical mattress 4.0 chromic gut sutures. The planned post-surgical relationship of the graft materials, the implants, and existing alveolar bone is illustrated in figure 2. Animals were provided antibiotic (Trimox), received a daily oral antimicrobial mouthwash (0.2% Chlorhexidine Gluconate) and a soft diet for 28 days. After a total of 6 weeks, the 6 dogs were euthanized and block sections of the mandible were made and fixed in 10% formalin. Four of the six constructs were evaluated for both the autogenous, MSC + HA-TCP and empty treatment groups. One of the six HA-TCP grafts remained intact surrounding the otherwise integrated implant for the entire healing period.
Histological procedures –Fixed tissues were processed for embedding in acrylic resin [21]. The embedded tissues were sectioned in the buccolingual direction to allow for assessment of the implants as well as the devices. Implants were bisected and additional sections were made from each tissue half. At least three sections were made through each implant and were evaluated after staining with Toluidine Blue by light microscopy.
Results
All 6 beagle dogs survived the surgery successfully and did not reveal clinical signs of infection or undue or extended discomfort associated with the procedures. The placement of implants was achieved without buccal or lingual bone dehiscence and primary stability was achieved for all implants. One construct (cell-free) was fractured upon placement. All cpTitanium implants were present at the 6 week time point. Implants associated with allogeneic MSC-containing grafts or with autogenous bone remained in a submucosal location. All implants that were not grafted and the implants grafted with cell-free constructs were in a transmucosal condition following 6 weeks of healing. There was no apparent inflammation of the mucosa at these implants.
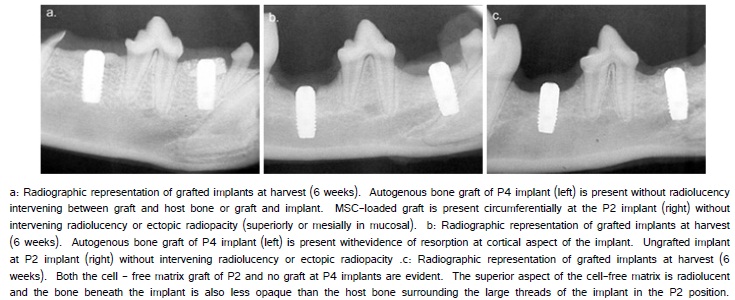
Radiographic evaluation of the mandibles following healing showed that the autogenous bone and MSC-matrix engrafted sites were intact and that bone formation occurred along the implant superior to the alveolar surgical margin (Figure 3a). In contrast, the cell-free matrix engrafted sites were devoid of the matrix and resembled the situation for the ungrafted implants (Figure 3b, 3c). There were no radiolucencies present at the bone interface formed against the 4.5 mm of the implant placed within alveolar bone. Other pathologic signs were not identified.
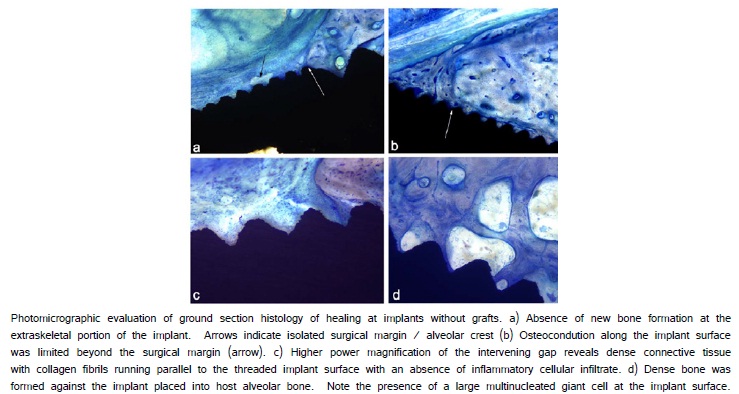
Histological evaluation of implants that were not grafted revealed the absence of osteoconduction from the superior surgical margins along the extra-skeletal implant surfaces (Figure 4a, 4b). A dense collagenous connective tissue formed at the implant-tissue interface (Figure 4c).There was limited cellular infiltrate located in the peri-implant connective tissue above the superior surgical margins. A direct bone-to-implant contact resulted at the implant along surfaces of the implant placed into the alveolar osteotomy (Figure 4d).
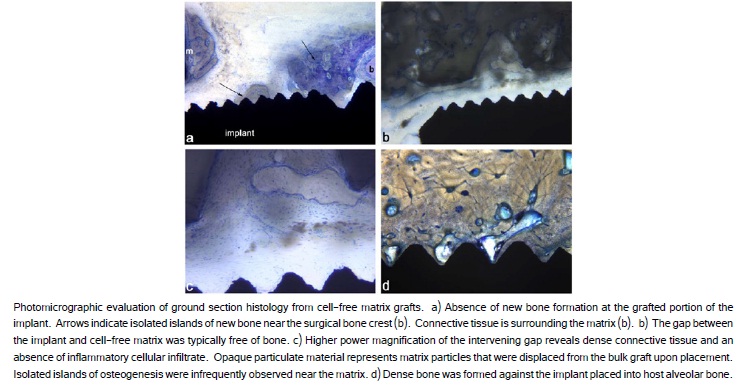
The evaluation of implants that were grafted with the cell-free constructs revealed a similar absence of bone formation extra-skeletally along the exposed implant surface. The gap between the implant and the matrix displayed no bone formation on the implant surface and one or two bone spicules (b) forming near the matrix (m) (Figure 5a, 5b). The peri-implant connective tissue in this region was largely devoid of polymorphonuclear lymphocytes or large numbers of mononuclear cells, or phagocytic macrophages (Figure 5c). The implant in host alveolar bone displayed a direct bone to implant contact (Figure 5d).
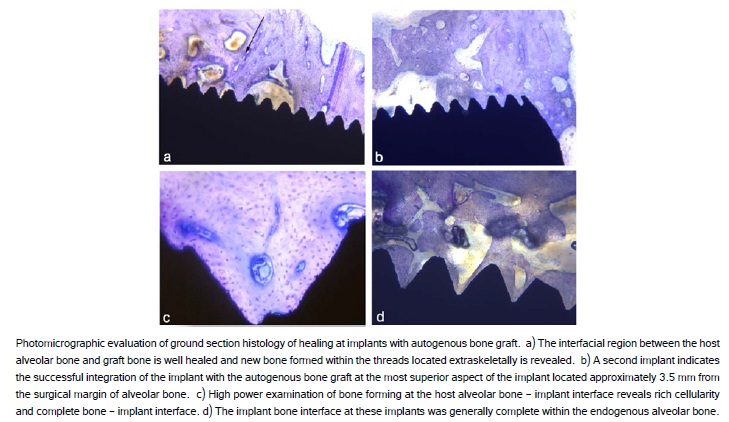
Histological evaluation of the bone-to-implant interface formed at the implants grafted with autogenous bone revealed successful osseointegration and vertical augmentation. The extra-skeletal region of the implant was opposed by the grafted bone or new bone formation. Because the autogenous bone was press fit around the threaded implants at surgery, any bone in the threads represents new bone formation following grafting (Figure 6a, 6b). Within host alveolar bone, new bone formation occurred within the threads and was observed along the majority of the endosseous implant surface (Figure 6c, 6d).
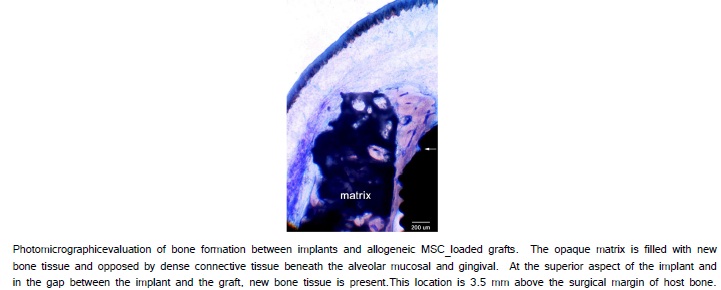
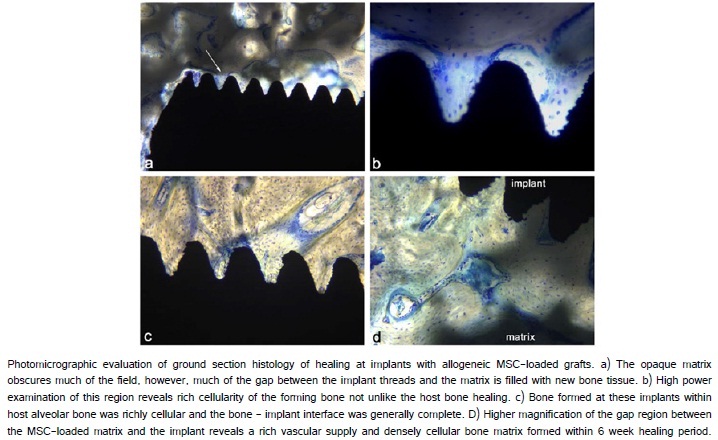
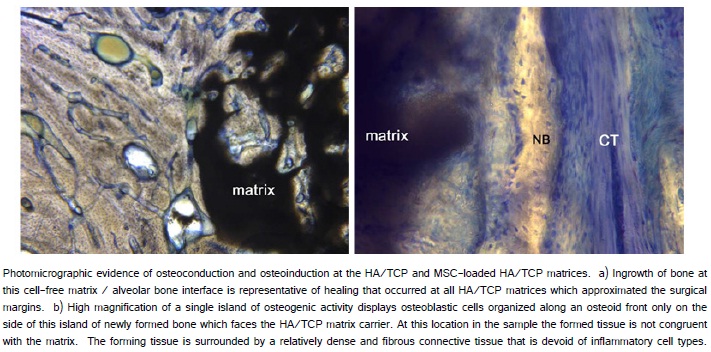
The histological evaluation of MSC-loaded HA/TCP engrafted sites revealed the maintenance of the HA/TCP matrix and the formation of new bone between the matrix and the extra-skeletal region of the implant (Figure 7). In the gap between the implant and the matrix, evidence of osteogenesis was observed in many locations spatially distinct from the surgical bone margin (Figure 8a, 8b). The implant surfaces were largely covered with newly formed bone both along the portion of the implant placed within alveolar bone and along the portion of the implant placed extraskeletally. Higher magnification revealed many sites where bone formation and supporting vascularization between the cpTitanium implant and the hydroxyapatite/tricalcium phosphate matrix (Figure 8d).
As expected, osteoconduction was not restricted to the MSC-loaded HA/TCP sites. Osteoconduction occurred within the region of all HA/TCP matrices opposing the surgical bone margin (Figure 9a). At the allogeneic MSC-loaded matrices, inflammation was not noted. Moreover, regions of new bone formation were observed superior to the MSC-loaded HA/TCP matrix beneath the alveolar mucosa (Figure 9b).
Discussion
Vertical bone augmentation at endosseous implants is a technically challenging procedure that, when successful, can contribute to the biomechanical and esthetic value of implant-supported dental rehabilitations [13]. Guided Tissue Regeneration is based, in a large part, on the creation of a protected space for host cell ingrowth and subsequent bone formation through the use of an exclusion membrane. Onlay bone grafts and distraction osteogenesis are alternative approached to vertical alveolar bone augmentation that present additional limitations to reproducible clinical success [10,12].The aim of this pilot study was to examine the feasibility of an alternative approach for concurrent implant placement and vertical bone augmentation using an allogeneic marrow-derived stem cell-based construct without the use of a barrier membrane.
Allogeneic marrow-derived stem cells were obtained from iliac crest aspirations from major histocompatibility loci mismatched donor animals and subsequent cell culture expansion. The results demonstrated that allogeneic stem cell-loaded devices supported the formation of a bone-to-implant interface along the entire vertical augmentation surface of the implants and along the bone-to-graft interface. Vertical augmentation was also achieved using autogenous bone. However, in the absence of allogeneic stem cells or in the absence of any matrix graft, bone regeneration failed to occur. This initial success with vertical alveolar bone augmentation at dental implants using an MSC-based tissue engineering approach suggests a number of avenues to improve or simplify current regenerative therapies in dentistry.
This pilot study in the beagle dog model demonstrates that allogeneic MSCs can be used to direct bone formation for the purpose of vertical bone augmentation at endosseous implants. A previous study of canine mandibular defect repair utilized DLA-mismatched bone marrow derived MSCs for successful bone repair [20]. Several studies suggest that allogeneic MSCs may evade alloimmune surveillance and suppress graft versus host disease. Recent direct comparision of allogeneic MSC versus syngeneic MSC engraftment in excisional wounds of mice demonstrated that allogeneic MSC engraftment did no cause inflammation and were effective in enhancing wound healing [22]. This pilot study did not investigate the contribution of the engrafted cells directly to bone formation. Histological evidence of immunological rejection or inflammation was not observed. There exists continued debate regarding the direct role of allogeneic MSC in wound repair. The present data cannot distinguish between possible direct osteogenesis or paracrine influences on local host cell contribution to the noted bone formation.
The approach chosen involved the simultaneous placement of implants and grafting without the use of a barrier membrane. The successful formation of bone at the engrafted MSC-loaded matrix/implant interface and the MSC-loaded matrix/bone interface demonstrate the potential for MCS-based tissue engineering to augment bone during dental and craniofacial regeneration procedures that involve titanium dental implants. The data indicate that a barrier membrane is not needed to exclude epithelial or fibrous connective tissue ingrowth at the surgical margin or the implant/MSC-loaded matrix interface.
Other investigations have demonstrated the potential value of a tissue engineering approach to vertical augmentation of alveolar bone at endosseous dental implants. A protective implant cover screw (“scaffold retention screw”) was used to maintain and protect a circumferential demineralized bone matrix scaffold placed around an SLA surface implant in the rabbit mandible. Histology revealed substantial supracrestal bone growth after 8 weeks [23]. As suggested in the present investigation, the authors noted that the titanium dental implant offers retention and stability of the circumferential regenerative device. This result reinforced previous success obtained using implants and protective hardware in a murine calvarial model [24]. Using a similar hardware approach in a rat mandible model, Wen et al. [25] delivered BMP—2 from a circumferential collagen / hydrogel to achieve extraskeletal/peri-implant osteogenesis. Thus, there appears to be growing appreciation for the combined use of tissue engineering devices and supporting endosseous implants for the purpose of directing extraskeletal, peri-implant bone augmentation.
This investigation utilized MSCs as an osteogenic agent. The present findings further expand the potential applications for cell-based tissue engineering of bone. Other tissue engineering efforts in dentistry involve the use of growth factors and matrix to achieve bone formation. The bone morphogenetic proteins have been most aggressively investigated [26]. BMPs have been used for ridge preservation and augmentation [27-28], sinus grafting [29-30] and for enhancement of osseointegration of endosseous implants [31,32].
BMP-directed bone formation is matrix dependent, similar to cell-based therapies. However, BMP actions are mediated by resident cells and may be dependent upon the numbers of cells present or recruited to the surgical site. This raises some question about the utility of BMPs for medically compromised, pharmacologically suppressed, or aging individuals where the numbers of available target osteoprogenitor cells could be limited or in bone defects of dimensions that are too excessive to be healed by BMP therapy alone [33]. While there is also significant research and development required to translate osteogenesis by MSCs to clinical bone augmentation procedures, some relative advantages may be attributed to cell-based approaches.
De novo bone formation can be supported by MSCs at a cpTitanium surface in an extraskeletal location. The implants used in this study have a TiO2-grit blasted surface and it is presently not clear what the role of the implant surface is on MSC-based bone formation. The histological results suggest that there is robust bone formation supported by the allogeneic MSCs and that MSCs may be further used for augmentation of bone formation between implants and bone in situations such as immediate implant placement into tooth extraction sockets.
Some possible challenges to a cell-based approach merit consideration. The harvesting of autologous tissues adds costs and possible morbidity at the donor site, however, the use of autologous cells averts some safety issues and host compatibility issues. The use of donor-derived cells or tissue allows off-the-shelf availability of implants and avoids additional procedures for the patient, but the potential risk of infectious agents or immune rejection represents challenges. It is possible to rigorously screen allogeneic donor cells and the success of donor-derived MSC-based bone formation indicates that allogeneic cells for human bone regeneration may be possible. In fact, donor-derived allogeneic MSC therapy without induced graft versus host disease or necessary immunosuppression has been successfully demonstrated in bone marrow transplantation secondary to high dose chemotherapy in humans [34].
In a similar beagle dog model, bone augmentation at endosseous implants was achieved using autogenous bone chips beneath exclusionary membranes [35]. The augmentation of alveolar bone surrounding dental implants was also achieved in a canine model using BMP-2 in a carrier beneath exclusionary membranes [36]. The potential advantages of the current approach for vertical bone augmentation include the simplification of the procedure using a single component for augmentation at the time of implant placement and the documented reproducibility of bone formation by the MSC. Further development of MSC-matrix relationships promises to improve the clinical handling characteristics of a circumferential device that permits simultaneous implant placement and vertical augmentation. By invoking the use of cryopreserved, allogeneic cells, a clinical scenario that involves a prepared, readily available cell-based tissue engineering product can be envisioned.
We are not aware of other investigations that have utilized cryopreserved and expanded bone marrow-derived MSCs for peri-implant vertical bone augmentation procedures. Others have succeeded in regenerating tissue-engineered bone (using MSCs and platelet rich plasma within b-tricaclium phosphate particles) for maxillary sinus augmentation or onlay grafting with simultaneous dental implant placement [37]. This early human investigation affirms the observations made repeatedly using various MSCs for the regeneration of bony surgical defects created in rat, rabbit and dog models [38].
Tissue engineering using MSCs can provide a variety of opportunities for dental and craniofacial bone regeneration. Tissue engineering can yield more complex constructs that control vascularization, matrix resorption, and osteoinduction of the included cells using recombinant growth factors, matrix molecules or gene therapy approaches [39]. Preceding these complex approaches, this specific application of the MSC has been evaluated to examine the potential for MSC-based constructs to perform vertical bone augmentation at endosseous implants. Merging of alloplastic technologies with biological approaches to regeneration appears to be a powerful approach to solving clinical problems associated with dental rehabilitation. Larger investigations using improved MSC and matrix combinations will continue to promote the use of cell and molecule based regenerative schemes in medicine and dentistry,
References
14. Caplan AI (1991) Mesenchymal stem cells. J Orthop Res 9: 641-650.