Research Article
Hans C Fledelius*
*Copenhagen University Eye Clinics, Rigshospitalet and Glostrup Hospital, Capital Region, Denmark
Corresponding author
Hans C Fledelius, MD, DSci, Copenhagen University Eye Clinics, Rigshospitalet and Glostrup Hospital, Capital Region, Denmark, Tel: +45 3545 2064; Fax +45 3545 2298; E-mail: hcfled@mail.dk
Received Date: 12thMarch 2015
Accepted Date: 13th May 2015
Published Date: 20th May 2015
Citation
Fledelius HC (2015) F x λ = v Dimensions in Ophthalmology. Enliven: Pediatr Neonatol Biol 2(3): 006.
Copyright
@ 2015 Dr. Hans C Fledelius. This is an Open Access article published and distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
F x λ = v, Dimensions in Ophthalmology- This was the title of the Jules Francois honor lecture presented at the XXV SIDUO meeting in Berlin 2-6 of July 2014 (Societas Internationalis Ultrasonica pro Diagnostica in Ophthalmologia). The event marked that half a century had passed since the ultrasonic society’s first meeting in 1964, also held in Berlin.
A survey of ultrasonographic investigations into human ocular growth and the impact of preterm delivery.
Elaborations on the Jules Francois Lecture, held at the XXV SIDUO Conference in Berlin, July 2014. With focus on human ocular growth and the impact of preterm delivery.
Contents
1. Preface
2. Dimensions in ophthalmology: Introduction
3. The University Hospital of Copenhagen Project 1959-61 on the Significance of Gestation and Delivery for the Health and Development of the Child.
4. Preterm?s eventual ophthalmic outcome: Restricted knowledge in the 1960?s. Myopia of prematurity, Refractive distribution.
4.1 Oculometry results at age 10 years (1969-72). Smaller eyes in preterms.
4.2 Refractive correlations, rules and exceptions. General remarks
4.3 Axial length and cornea
4.4 The lens
4.5 Discussion and Summary
5. Will the preterms ever catch up? The final part of the human growth curves
6. The Copenhagen Project 1959-61, ophthalmic data followed up at age 18-19
6.1. Sorsby: eye growth till age 13. IOL-Master a new tool.
6.2. Revised end phase of growth curves
6.3. Eye growth seen in relation to congenital cataract and to adult age refractive surgery
7. How early is ocular growth deficit in preterms obvious?
8. Neonatal eye data. Preterm?s in general.
8.1 Advanced retinopathy of prematurity, on the threshold to retinal ablation therapy.
8.2 Intrauterine growth retardation. High frequency ultrasonic biomicroscopy (UBM) and optical coherence tomography (OCT)
9. Summary
Preface
F x ? = v Dimensions in ophthalmology- This was the title of the Jules Francois honor lecture presented at the XXV SIDUO meeting in Berlin 2-6 of July 2014 (Societas International is Ultrasonica pro Diagnostica in Ophthalmologia). The event marked that half a century had passed since the ultrasonic society?s first meeting in 1964, also held in Berlin.
Focus of the lecture was on the ultrasonically determined dimensions of the eye and the widening of our knowledge regarding optical parameters and their interplay, from fetal stage and on. This includes what happens from intrauterine conditions and past delivery to adult age. Here this is filtered through the mind of the lecturer, who has been active in ultrasound since 1968. The author?s aim has been to specify and discuss some of the stepping stones on the way, outlining a development that has literally extended the dimensions of ophthalmology beyond mere ocular size aspects.
Introduction on Dimensions
The eye always challenged lay and learned. It creates images, whose perception constitutes visual awareness and navigation in the world around us. The eye has further been regarded the residence of the soul.
Many concepts still valid were founded on Leonardo da Vinci?s (1452-1519) anatomy studies and on his creative thinking as a polyhistor. Some fundamental mistakes regarding the imagery were later corrected by Johannes Kepler (1571-1630), as based on astronomical observations by Tycho Brahe (1546-1601). The ground was laid for the physiological optics of the 19th century. The advanced ray-tracing techniques of today represent a continuation of the tradition.
Equally important for scientific dimensions was the eventual rejection of the myths and theories from the dark middle age. A millennium?s confessional tradition should finally be challenged by the thinking of a new era. Based on studies of the brain, the anatomist Nicolau Steno (1638-86) stated: watch Nature, describe details with precision and free of bias, found theories, test hypotheses and see whether results are reproducible. ?Most beautiful is what we do not (yet) know?.
Modern ophthalmology had its onset with the ophthalmoscope: a new subspecialty was sorted out (von Helmholtz 1821-94). It appealed to a small segment of doctors who took interest in the mini-world as previously concealed behind the dark pupil. Reflected light could be visualized, funds findings described, and diseases characterized. However, it was not possible to get beyond structures that visible light could not penetrate.
In the 1950?s ultrasonic techniques made it possible to bypass tissue surfaces in vivo. There will be reflection from surfaces that separate media of different acoustic density, but part of the sonic pressure front will further precede into the tissue. Reflected ultrasonic energy from deeper structures is amplified and visualized on a receiver screen, and comparison can be made between sonic profiles in vivo and real tissue microscopy.
Diagnostic ultrasound is now an established and indispensable non-traumatic chair side discipline in eye clinics all over the world. For morphological evaluation of the eyeball and its disorders it adds decisively to the visually based methods, and even gives more detailed information than CT and MRI scan. In the hands of experienced examiners useful guidance may further be obtained regarding pathology in adjacent orbit and sinuses. Here, however, CT and MRI yield more specific information and are usually needed for evaluating orbital pathology and planning of surgery.
Ultrasound measurements have added another important dimension to ophthalmology. Scientifically, we benefit from a widely increased knowledge about natural variation and correlation regarding ocular parameters. From a practical point of view, a brief mention only of the millions of cataract patients worldwide, who can have their intraocular lens power tailored and enjoy quitting their annoying ametropia after surgery [1-5].
This author enjoyed a lifelong fascination of both diagnostic and oculometric aspects. A specific interest attached to ultrasonic oculometry, with a flow of studies performed back from the pioneering period of the 1960?s. This commitment underlies the following elaborations on items that will focus mainly on dynamic aspects of refraction and eye growth. It all started with a scholarship in 1968.
The University Hospital of Copenhagen Project 1959-61 on the Significance of Gestation and Delivery for the Health and Development of the Child
University hospital obstetricians and pediatricians followed a birth cohort comprising 9006 children delivered in the maternity departments [6]. The Copenhagen University Hospital clinics took care of complicated pregnancies and deliveries, but also of so-called ?illegitimates?, a designation for extra-marital pregnancies. Here they comprised 30% of the cohort, against a percentage in the population of 7-8%. They were considered as mainly healthy pregnancies, in contrast to the females that had been hospitalized for expected or obvious pathology.
From 1969, a University scholarship made an ophthalmic study possible. Eventually, hyper oxygenation had been clarified as underlying the unhappy United States epidemic of blindness among prematures in the 1940?s. With due measures taken ophthalmic landslides soon became obvious, in developed societies. Having achieved this general state of affairs from about 1960, we formulated a Copenhagen project of elucidating the influence of the ?prematurity trauma? upon the subsequent development and function of the eyes. This was done by way of a broad ophthalmic status around age 9-11 years. At this age eye development is considered almost done, and most children can co-operate for examinations.
Demographically, we invited all preterm survivors of a birth weight <2000g (n= 336), and 90% were examined. 28 % scored <1500g for that period considered a low birth weight. The controls were randomly selected among those fulfilling a birth weight criterion of 3000-4000g; their final attendance was 74%. The preterms actually seen numbered 302, the term controls 237. The mean age was 10.2 years in both groups.
From the mandatory Danish registration of visually impaired children it was known that 3 of the included preterms were blind (2 from retrolental fibroplasia, and 1 from brain damage). This means that 99% of the study group seemed to have normal or fair vision, and deviations from ophthalmic norms were expectedly minor. To gain new knowledge, ultrasound oculometry was included as an additional procedure, provided accept from parents and children. Around 1970 several studies had reported on eye dimensions in neonates in general [7-10]. The only study that included prematures indicated a full catch up regarding preterm?s eye size within the first year of life [11].
A full report is presented in my thesis for the doctorate ?Prematurity and the Eye? (Acta Ophthalmologica1 976, Suppl. 128) [12]. A thorough discussion was given of the immersion ultrasonic measurement method, now considered primitive, and not of great esteem on behalf of the children. Axial measurements were obtained in 191 preterms and in 159 controls, and right eye oculometry values were elected for statistical evaluation.
Preterm?s Eventual Ophthalmic Outcome: Restricted Knowledge in the 1960?s. Myopia of Prematurity, Refractive Distribution
The literature contained sporadic reports on early childhood myopia in preterm survivors where initial retinal reactions, if and when recorded, had regressed, and left the eyes with visual function [13-16]. Besides visual ability, the first question regarding the Copenhagen data thus was about refractive distribution. 13.3% of the preterm?s eyes were myopic, compared to 9.2% in the controls, and mainly it was low myopia ?as usual?. The slight preterm surplus of myopes seemed to be due to a fraction of children, who had early onset of the myopia. This was labeled pre-school myopia, or myopia of prematurity (MOP), and 17 out of 27 such eyes had myopia exceeding 3 dioptres. In contrast, all 53 preterm eyes with school myopia at the age of 10 years were numerically below this value. In accordance with western experience, among the 237 controls only one child had pre-school onset recorded. Refractions in the most normal area (between 0 and +1.9 D) occurred in about 73% in both groups. Nor did the hypermetropic tails of the distribution present significant deviation [12].
Oculometry Results at Age 10 Years (1969-72). Smaller Eyes in Preterm?s
(Table 1) presents main findings in the full study groups. Around the age of 10 years, the preterms had significantly shorter eyes, the corneas were more curved, and lenses were thicker. The same was evident when comparing only the emmetropes, the refractive subgroup which is usually considered the least affected/most normal (here given by refraction 0 +0.9 D). The emmetropes presented mean axial lengths of 23.08 and 23.50mm in preterms and controls, respectively; the corresponding corneas had 7.67 and 7.85mm as mean curvature radius, and lens thicknesses were 3.61 and 3.56mm. All three differences between groups were statistically significant (t-test, P < 0.01).
|
|
Preterms (n = 191) |
Term controls (N = 159) |
|
Refraction (D) |
+0.63 (2.25) |
+1.05 (1.35) |
|
Axial length (mm) |
23.01 (0.97) |
23.37 (0.82) |
|
Ant .chamber (mm) |
3.82 (0.25) |
3.82 (0.25) |
|
Lens thickness (mm) |
3.63 (0.92) |
3.58 (0.77) |
|
Corneal curve. radius (mm) |
7.69 (0.30) |
7.87 (0.26) |
Table 1: 10-year cycloplegic refraction (in dioptres), axial ultrasound measurements (in mm), and corneal curvature radius (in mm) in right eyes of preterms and controls, from the 1959-61 cohort, given as mean values with standard deviation.
(Table 2,3) specifies the findings among those with myopia, subdividing the preterms by either early MOP, or the later variety of juvenile or school myopia. The eyes with myopia of prematurity clearly sorted out from the rest. Despite a higher degree of myopia, the eyes were considered ?short for myopia?, and the arrested anterior segment features were even more evident than apparent from (Table 1). Compared to the other preterms, a lesional component was further suggested from the significantly lower best corrected visual acuities in the MOP subgroup. Thess contrasts to ?ordinary? juvenile myopia, where best corrected visual acuities often are above norm. It can be added, that 97.4% of the preterm subjects had a best corrected visual acuity (binocular, in decimal notation) above 0.5, and only one percent was blind.
|
|
Myopia of prem n=21 |
Juvenile, in PT n=19 |
Juvenile, term n=22 |
|
Refraction (D) |
-5.15 |
-1.0 |
-2.27 |
|
Axial length (mm) |
24.38 |
23.90 |
24.32 |
|
Anterior chamber (mm) |
3.77 |
4.02 |
3.95 |
|
Lens thickness (mm) |
3.79 |
3.64 |
3.58 |
|
Corneal curv.radius (mm) |
7.57 |
7.70 |
7.73 |
Table 2: Same parameters as in Table 1, here separated for myopic eyes of three categories: myopia of prematurity (measurements from 22 eyes), juvenile myopia in preterms (21 eyes) and myopia in term controls (19 eyes), mean values only.
| ALACD | ACD | LT | VL | Crad | |
| Refraction (D) | -0.62 (-0.76) | -0.34 | -0.34 | -0.56 (-0.77) | 0.2 |
| Axial length (AL, mm) | 0.44 | 0.44 | 0.95 (0.97) | 0.37 (0.44) | |
| Ant chamber (ACD, mm) | -0.31 | 0.22 | |||
| Lens thickness (LT, mm) | -0.26 | ||||
| Vitreous length (VL, mm) | 0.39 |
Table 3: Correlation between optical parameters in two age homogeneous Danish series: In 10-year-old full terms (N= 159 right eyes, Fledelius 1976), given by parametric Pearson correlation coefficients (r values). For comparison: non- parametric Spearman rank correlations, in parantheses (rS values), from 26-year-old high myopes (58 eyes, n = 29; Goldschmidt et al. 1981). Crad = corneal curvature radius (mm)
Refractive Correlations, Rules and Exceptions. General Remarks
Axial Length and Cornea
Donders emphasized that markedly myopic eyes were long and also had an increased risk of ocular morbidity [17]. From the early 1960?s, ultrasound could specify the elongation, and also pave the way for a mathematical expression of the strong correlation between axial length and refraction. In particular this held within what Sorsby labelled correlation ametropia, often conceived as refractions between +7 and -7 D [18]. The term signifies that the refractive components vary within normal, but their combination, when unbalanced, explains the refractive error.
Cornea ranks second in correlation. At delivery cornea is peaked, and refractive power is high, but it adjusts to final measures of curvature/ refractive power within 1-2 years. Eventually, a longer eye will typically have a flatter cornea (curvature radius a higher value), and correlation coefficients are often at a level of r = 0.4-0.5. The combined effect will be a lower total refractive power, partially to compensate for the effect of childhood elongation towards myopia. As an indirect consequence, in most studies corneal curvature (or power) does not correlate with refractive value, but only with the axial length. This is illustrated by (Table 4), which shows correlations between main optical parameters in young Danes. Age is eliminated as a confounder within the two series: The 10-year-olds of the thesis study and the 26-year-olds in a longitudinal Danish cohort study of high myopia [19,20]. Anterior chamber depth and lens factors such as thickness, surface curvatures, and refractive indices of sub-compartments, certainly should be recognized in ray tracing analyses. Empirically, however, their impact seems minor when relating to total refraction.
In excessive ametropia, outlier values may underlie the refractive error. In particular this manifests as elongation in high or pathologic myopia. Corneal flattening is less obligate when myopia exceeds 7-9 dioptres, or axial length values are above 26-28mm, respectively [20-23]. Probably, heredity has a role to play, in particular in the cases where corneas are peaked.
|
|
Preterms |
Full terms |
|
Refraction (D) |
+0.40 |
+0.31 |
|
Refr.changeage 10-18 (D) |
0.86 |
0.74 |
|
Ant.chamber depth (mm) |
3.91 |
4.0 |
|
Lens thickness (mm) |
3.66 |
3.54 |
|
Axial length |
23.30 |
23.73 |
Table 4: Oculometric parameters at age 18-19 years in 70 preterms and 67 full term controls, male and female data pooled, presented as mean values
The Lens
Refractive correlations further have a natural focus on the lens. Its life-long addition of new lens fibres currently adds to its volume, with an about 0.2mm axial thickness increase per decade in adults, a figure duly established by ultrasound [22]. The adult anterior chamber correspondingly reduces depth, approximately by half the lens thickness increase value. In shallow chambers, eventually this can have the consequence of triggering angle closure incidences.
A key role on refraction would be expected for lens factors, but based on regression mathematics the impact seems almost absent. Adult age refraction is usually stable over decades, despite the continuous increase of the lens thickness and the relative anteposition of first surface for lenticular refraction. Sub-cortical opacities eventually become frequent, often from age 60 years. Typically this slowly developing type of cataract is accompanied by reduced lens thickness [24], and the shift to slight hyperopization when past the age of 50 years usually continues [25-29]. As for cataract morphology, the myopizing nuclear type seems less frequent. The relevant direct power measurement in the context should be phakometry, but this seems to be a difficult (and not much used) procedure. Instead, many authors do the indirect shortcut of standard intraocular lens calculation, here ?backwards? from the given refractive value, for assessing factual lens power. According to literature, the general trend in adults is a decrease of lens power by age, from teenage and on [23].
In early life, correlational aspects appear at least as interesting. When still in utero there is a physiological spherophakia, which explains the transient myopia of infants delivered before term. Usually the conversion to hyperopia in the preterms is paralleled by the physiological lens flattening. Most such eyes thus simply join those of the term children, with their early hyperopia, and soon they will be the subject of emmetropization. The preterms metabolic turmoil, however, disturbs the programmed growth process, at least in some, and arrested features will manifest. The thicker lens and the flatter chamber will, alongside with the more curved cornea, optically contribute to the myopia of prematurity, and not as usually compensate [12,30-32]. The other way round: the physiological lens flattening up to teen age counteracts the optical effects of axial elongation and contributes to emmetropization.
Undoubtedly, the correlational puzzles include genetics as well as environment. At the SIDUO II in Brno in 1967, Gernet presented a fascinating mechanical theory as related to early lens changes [33]. The zonular suspension ring increases in size by age, and increased zonular tension would explain the lens flattening of childhood. Provided meridional and axial growth of the pars plan region up to ocular growth arrest, this might fit into the pattern of thinnest lens at age 12-13 years [22,34]. It is also in accord with the subsequent ?adult? increase of thickness as well, though now being due to the continuous lens growth. Further, small hyperopic eyes generally have thick lenses, and long myopic eyes flatter lenses.
Discussion and Summary
Summing up the new dimensions in ophthalmology as achieved from the Copenhagen University cohort study 1959-61:
? There was significant evidence of a general arrest of eye growth in the preterms when examined about the age of 10 years, despite good vision in almost all, and an overall normal clinical eye status in most. The preterm deficit regarding axial length and corneal curvature radius equaled the size difference according to gender. From delivery to adult age, boys eyes are larger than girls and have flatter corneas, however with no size-related differences in eye functions.
? Parallel size differences according to preterm/term (as also to gender) were obtained when analyzing body height, inter pupillary distance, cranial circumference, and transversal corneal diameter. Thus, around age 10 years there was no general catch up.
? Myopia is predominantly due to axial elongation of the eye [12,17,21,22,33,35-37]. This is evident also from parallel vitreous length findings, all to suggest myopia as being almost exclusively a posterior eye segment event. However, in the 1976 thesis it was emphasized that ?failing adaptation of the structures in the anterior chamber seems to have had an influence upon the refractive value, particularly in the pre-school myopes?. This distracts the customary primary attention from a vulnerable posterior eye segment in preterms at risk to a holistic inclusion of the anterior eye segment as well. Apparently no wonder, regarding the rapid changes in normal anterior chamber parameters during the first 1-2 years of life. In particular, this is given by the keratometry data. In relation to MOP, the anterior segment parameters do not emmetropize, but add to the myopia, as relative index features.
? The corneal curvature findings raised another interesting discussion [12,25]. As for the association between refractive components, the correlation between axial length and corneal curvature radius numerically ranked second only to the dependence of refraction on axial length value. To exclude preterm related confounders, we here analysed only the term group. The flatter cornea in longer eyes is in agreement with other contemporary reports on population data, and with more recent reports as well. Timely, however, the two main parameters behave differently. The axial elongation of myopic eyes continues as long as the myopia is developing, which may happen throughout teenage years. Eyes already refractively stable at early teenage usually are considered fully grown. On the contrary, the apparently definitive corneal curvatures are established already from age 2 years. The eventual positive correlation between adolescent and finalized adult axial length and the early established corneal curvature radius thus may suggest a hereditary component. Despite obvious environmental factors in generating myopia in the population, the basic DNA messages seem to co-determine an apparently balanced oculometric end result. If ?environment only?, the established flatter corneas in the longer myopic eyes would not be a so constant finding in oculometry series, whether preterm or population. Data from our age 18-19 year longitudinal follow up later unanimously indicated unchanged corneas regardless of adolescent axial elongation. The general juvenile myopic blowing up the eye is primarily a posterior segment event and does not include the cornea. This opinion has support from longitudinal data in other Danish large scale studies [20,37-39].
In Conclusions
Evaluating preterm data, the level of neonatology service should always be considered. For the present data we are definitely historical, dating back to ?survival of the fittest?. Supplemental oxygen was given mainly by a funnel near the infants head. Retinopathy of prematurity (ROP) - as later to be defined [40] was probably triggered in some, but simply could not manifest in the many infants who died during the first 4 weeks. Based on the 1960 general health system in a post-war western country with limited access to developing intensive care neonatal facilities, we can safely conclude that surviving healthy looking preterms had not caught up at the age of 10 years.
We recently updated the discussion on ocular function and morphology in a 2015 review article on preterms, which has taken current therapeutic advances into consideration [41]. In general, the two main refractive trends are still to be emphasized. Relatively high myopia often develops early in preterm survivors, often in proportion to the early stage of ROP [42-46] and the oculometric features in MOP have remained on line with the original Copenhagen findings [12,30,31,37,47].
Will the Preterms Ever Catch Up? The Final Part of the Human Growth Curves
Reflecting Copenhagen conditions about 1960, the growth of the eye thus had not caught up with full term controls around the age of 10 years. The same held for body height, for cranial circumference, and for interpupillary distance measurements. With the close anatomical relation to the brain, the interpupillary distance may be regarded a parameter ?in between?, seen in relation to the established growth pattern of brain & eye as compared to general bodily growth and sexual maturation. The cerebral growth pattern has its most marked increase over the first few years, with an ongoing deceleration until age 12-13. Body height and weight mainly increase evenly during childhood and the teens, with the definitive height attained at age 15-16 years in females, and age 17-18 in the young male.
The high mortality of the very preterm infants emphasized their need of more care than for the full terms, but other basic differences between the two main Copenhagen groups were not obvious. In particular, distribution on social classes appeared similar; all pregnant females had free support from the health system, and the preterms lagging behind could not be so explained. On this background we added the question about delayed only vs. permanently affected development in the preterms. Hypothetically, this left the possibility of catching up for instance at teenage level. The question could be addressed by examining the 10-year-olds again when definitely having finished general growth.
The Copenhagen Project 1959-61 Ophthalmic Data Followed Up at Age 18-19
137 of the original 539 10-year-olds attended for re-examination at age 18-19 years (70 preterm and 67 full term) [48-50]. With the time allocated, full randomization was sacrificed for the follow-up. A mild selection for invitation was operative, favoring a significant contribution of the oculometrically interesting myopes. At both ages, refraction was based on retinoscopy and subjective trial, after having relaxed accommodative tonus by tropicamide 0.5% eye drops given twice.
Analyzing axial eye growth between age 10 and 18-19 years, the eyes were first grouped according to change in refraction. Stable refraction was given as less than half a dioptre change during adolescence (n= 49 eyes); slight refractive changed enoted an 0.6-1.5 D relative myopization (n= 61 eyes); moderate change ranged 1.6-2.5 D (n= 24 eyes), and a change above 2.5 D over the period denoted advanced myopization (n= 23 eyes). Axial elongation averaged 0.45 and 0.5mm in the two first groups (those of no or low change). They comprised 70% of the follow-up sample, and a figure of 0.4-0.5mm was regarded the basic final elongation. The two sub-groups with higher myopization had an average axial elongation of 1.15 and 1.61mm, respectively. A few outliers marked the full refractive range in the follow-up sample as +8D to -16 D; axial lengths ranged 20.3-28.7mm. Highest myopia occurred in the myopia of prematurity (MOP) subgroup. Presenting a median juvenile change of only 1.2 D, however, those with MOP had progressed less during teenage than those with ordinary juvenile myopia. The latter actually showed median changes of 1.7 and 2.5 D, in preterms and controls, respectively.
To minimize confounding effects of refraction on eye size, the supposedly most normal refractive mid groups of emmetropia at age 18 years were selected for evaluating the issue of lagging behind. (Table 4) shows that the previously stated size deficit of the preterms was maintained. We here emphasize the mean values for axial length and corneal curvature radius of 23.3 and 7.67mm vs. 23.73 and 7.90mm, in preterms and controls, respectively. In both genders there still remained a body height deficit of 3-4cm in the preterms, and cranial circumference lagged 1 cm behind. It should be commented, that the full 8 year interval between examinations did not allow a more precise estimate of interim end points for growth.
Sorsby: Eye Growth till Age 13. IOL Master a New Tool
Sorsby?s statement of eye growth until age 13 has stood the test of time over two generations [51]. With ultrasound oculometry only on its way, his analyses were based on physiological optics. Axial lengths were calculated from keratometry, phakometry, and cyloplegic refraction.
Ultrasound biometry soon added the multitude of data that disclosed the true biological variety of optical parameters, and their interplay as well. However, with an estimated ±0.2mm error on axial measurements the method was not suited for assessing the small annual differences that mark the deceleration of growth towards its end phase. For long, therefore, the ?growth till 13? concept was not seriously challenged. Probably it was also the excursions of single measurements that explain why Zadnik and coworkers did not proceed from cross-sectional analyses in their important 2004 childhood study, which also contained longitudinal data [52].
This limitation due to method now seems overcome by the IOL Master equipment, which is based on an infrared interferometer based technology. It is characterized by an impressing reproducibility of measurements within ±0.01 mm in many, and ±0.02 mm in most. Further it has the advantage of no touch, and of ensuring optical measurement to the macula through fixation of the red aiming beam. We published data in 2014 that indicated continuous elongation also past the age of 13 years, though decelerating and on a rather small scale. Physiologically this can be taken as an indication of a retained potential for tissue plasticity. Probably this is based on directional changes in eye wall modeling associated to the current ana- and catabolism of tissue, here primarily the scleral coat.
Obviously the item is of importance not only for juvenile myopia that still progresses, but also for adult onset myopia. This refractive state is more common than usually apparent from textbooks and may account for 20-30% of the myopia segment in adult western populations [53,54]. It often occurs among university students when passing the age of 20, but also among the lay; according to recent hypotheses in myopia research it is driven by aberrations related to imaging, and mediated by tissue factors. The basic paradox seems to be that adult eyes can re-uptake axial growth after apparently being outgrown and having achieved stable emmetropia, as adolescents. Adult onset myopia shares the feature of axial elongation with childhood and juvenile myopia. Many such ?student myopias? remain of a low degree, but in my files I have several cases eventually aged 50-60 years who had progressed down to -8 D, and presenting axial lengths around 27mm.
Revised End Phase of Growth Curves
The body height is easy to measure, and with no obvious confounders. Healthy children present a mainly even annual increment, often ending with a pubertal spurt phase. Apparently, this late teenage growth event leaves the size of most eyes unaffected.
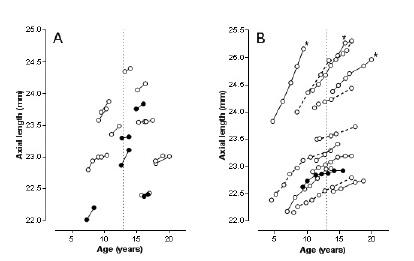
The growth curve of the eye is exponential and asymptotically approaches zero when passing the age of 10-12 years [12,55,56]. When population based, an increasing share of juvenile myopia will, however, blur the final plateau as otherwise expected [9,52,57]. Clearly, a fixed end point will have validity only for stable eyes, as typically seen as a mid-group in western societies, with their customary ?leptokurtotic? share of eyes clustering around emmetropia and low plus refraction. In our recently issued longitudinal study on axial growth we therefore aimed at keeping eyes with axial elongation due to myopization out of calculations. Our focus was on afictive median eye in the population, the refractively stable eye with emmetropia. We recently added extended teenage data for five random emmetropic males out of those already included [56], however without leading to changes in conclusions. (Figure 1) shows our slightly revised longitudinal growth diagrams. The graph is now based on a total of 86 annual axial length increments [n= 31 subjects, separated in those having a maximum of 4 individual measurements (A, at top) and those with up to 8 annual value sets (B, bottom)]. The five dotted curves represent the added/revised courses, and the vertical line that marks age 13 underscores the continued growth also after this age. Only two teenagers presented zero as annual ?increment?, and there were no negative differences. However small, a negative value would unanimously have indicated stop of growth, whereas zero could be due to instrumental error only.
Figure1: Individual emmetropic growth curves based on annual axial length increase, in 17 subjects with up to four IOL-Master measuring sessions (at left, A) and 14 with 5-9 longitudinal measurements (at right, B). Open circles boys, black circles girls. *) denotes three subjects who eventually acquired borderline myopia. The dotted curves mark the five teenage cases with recent measurements added, seen in relation to a previously published graph (Acta Ophthalmol 2014; 92: 259-264). Age 13 is given by the vertical dotted line.
Summary
All considered, the results support ?growth also after 13?, though mainly as a biological event. The final basic ocular growth appears minimum, and of no obvious practical importance. However, it suggests an eventual role for ?dormant? eye plasticity.
Eye Growth Seen in Relation to Congenital Cataract and to Adult Age Refractive Surgery
Implanting intraocular lenses in neonates with dense congenital cataract presents two challenges. The first one is technical and with regard also to the frequent complications that occur within the first half year of life. Therefore a less dense cataract is generally hoped for, which might allow postponing intraocular surgery to age 1-3 years. This approach will also facilitate determining the pseudophakic lens power best suited for the child?s developmental needs, which includes best optical fit as amblyopia profylaxis. As for best choice of intraocular lens, for now and the future, it is easier to make a qualified guess when the first part of the growth curve can be safely evaluated [58]. In particular it is important to sort out the eyes with microphthalmic features, as evident in some eyes with congenital cataract. Such eyes do not follow the usual growth curve [55,58].
Another context is the expanding discipline of refractive surgery in adults, where it is usually myopia that is dealt with. Here it is generally stressed, that the client should be at least 20 years old and with stable refractive value. However, exceptional myopia cases show a slow progress over decades, and the population segment of ?plastic? adult onset myopia should also be considered. This entity clearly shares features with insidiously progressive myopia. The juvenile myopia of a young colleague of mine had -5 D as apparently stable adolescent refractive endpoint in her early twenties. At age 25 years she changed from a desk job to studying medicine and soon progressed to a permanent level of -8 D.
Admittedly, the reservations here touched upon apply only to a minority of cases, but refractive change of adult age should not be ignored. Thus, silent late myopization may explain why some patients with corneal refractive surgery eventually lose their initial enthusiasm with the optical result.
How Early is Ocular Growth Deficit in Preterms Obvious?
In my basic cohort 59-61 study the expression ?the trauma of prematurity? was used [12]. The aim was to set focus on associated deviations, as evident from a subsequent mapping of development and function of the eyes. Semantically, the environmental label might imply an absence of prenatal factors, but this is seldom the case. Very preterm delivery per se always indicates some general pathology. One example could be small for gestational age (SGA), as apparent in one third of such pregnancies. This is synonymous also with intrauterine growth retardation (IUGR), but moreover a multitude of pathologies are conceivable, environmental as well as hereditary. Whatever the mix, tissue growth factors and cytokines seem instrumental.
In the preceding I stressed that the growth deficits indicated in the early thesis study were valid for that sample and that era. However, a follow-up study of ROP performed two decades later in a Danish region outside the capital generally seemed to confirm the lagging behind in eye size [31,59,60]. In the early 1980?s the ROP classification had just been elaborated [40], and the principles for surveillance for ROP had been implemented for the new sample now under study. The follow-up comprised children of an average age of 8.7 years (range 7-10 years); 4 out of 28 were blind due to ROP, but the remaining 24 were recorded with only mild and reversible ROP. In our greater Copenhagen vitreo-retinal clinic cryo-treatment was not established until a few years later and had not been available for the advanced ROP cases of the new series under study. Further support for affected growth has been given by later analyses of myopia of prematurity, including the almost pathognomonic evidence of arrested anterior eye segments [30,31,37,60]. Obviously this has to be an early event, but data did not allow an exact timing.
Neonatal Eye Data. Preterm?s in General
In the same regional hospital, another project included 101 preterm neonates- all surveilled for ROP neonatally and 25 full term controls [61,62]. Usually 8-12 weeks had passed from preterm delivery to term (week 40), and the expectation was that retarded growth might have manifested already in the early neonatal period. Evaluated from serial early funduscopy, the preterm group under study did not appear clinically heavy; 75 had no ROP observed and 26 reversible ROP, stage 1-2. Gestational ages at delivery ranged 25-34 weeks, birth weights 728-2480g. Early around term growth curves could be compared, but there was no clear separation regarding axial length. A term axial length of 17mm was suggested for both groups, however with a within-group trend of shorter eyes when preterm GA was <30 weeks [63]. All considered the general conclusion had to be: no safe evidence of a general preterm growth arrest that early. Contemporary Irish data however suggested a growth deficit, though based on series that appeared more loaded by ROP [42,43].
For plain historical reasons we have no knowledge about neonatal retinal status in the preterms of my basic 1959-61 cohort. In the very theory, they might have shown growth delay already at gestational age week 40, cf that growth arrest appeared a full group feature at follow-up, and retinal reactions cannot be excluded. As judged from the subsequent low score of MOP, however, early ROP is not very likely nor was it suggested from the scarcity of cicatricial fundus changes observed at age 10 years [12]. Comparing the data, cautiously it can be stated only that no answer (to ?arrest how early?) was provided by the basic Copenhagen University Project data [6,12] nor could the regional neonatal preterm sample examined 23 years later specify growth arrest around normal term (week 40+) [63]. Clearly this was based on a preterm sample where regular surveillance for ROP had indicated only mild early fundus findings, whereas the above Irish series suggested dependency on ROP degree, the higher stages in particular.
Advanced Retinopathy of Prematurity, on the Threshold to Retinal Ablation Therapy
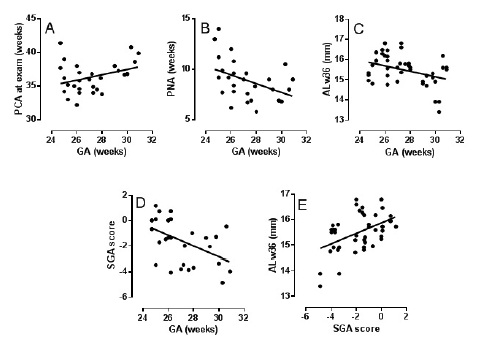
On the contrary, the final Danish sample of neonates yet to be introduced is indeed heavy. On a national scale, treatment for threshold ROP was (and is) centralized to the vitreo-retinal clinic of Rigs hospital, Copenhagen. All advanced cases of ROP are examined under general anesthesia of a specialized team prior to the decision regarding continued observation vs. retinalcryo ablation. During the years 1997-2002 we added ultrasound oculometry as part of the work-up in the operating theatre (Sonometrics 400, solid tip 10 Mc transducer), when conditions allowed. In my experienced hands the examination was performed within 1-2min. The median age of the preterm neonates was 36.2 weeks, and axial lengths from 51 eyes (n = 28) were adjusted to this age by way of locally established weekly growth rates (cf. ALw36 in (Figure 2); Fledelius & Fledelius 2012) [47]. The inclusion criterion for all eyes was threshold ROP (TROP), and randomization to ± treatment was regarded unethical.
Figure 2: Five selected scatter plots and regression lines as based on 51 eyes with threshold retinopathy of prematurity (n = 28), cf. text. Gestational age on abscissa in A-D, compared to post conceptional and postnatal age (A,B), to adjusted eye ocular axial length (ALw36; (C), and to intrauterine growth retardation, as expressed by a small for gestational age standard deviation score (D), E shows adjusted eye size (ALw36) as related to small for gestational age (IUGR).
In the inherent lack of a control group, we first compared with our previous (less loaded) neonatal data, and found a significant 1mm relative shortening of the TROP eyes. Next, two-by-two comparisons of parameters were done by way of regression statistics. Five such sets will be discussed in more detail, of which the two first primarily confirm previous knowledge [64]. The trends were (Figure 2A) that a lower GA at delivery also meant a lower post-conceptional age (PCA) for safe recording of threshold disease as eventually having developed this far (TROP), and (Figure 2B) that this was developed earlier reckoned by postnatal age (PNA), the higher the GA at delivery. Expressed by theoretical on-regression-line eyes of gestational age week 24 and week 30 respectively, the 24 week subject with advanced ROP reached the TROP stage at a PCA that is 2 weeks lower, whereas a 3½ week higher PNA value is needed. Both relationships express, that several weeks have to pass after delivery, where the ?trauma of prematurity? exerts its influence, until eventual treatment level of ROP is observed. The added interpretation, however, is that the preterm infant generally requires a certain degree of development prior to onset and progression of ROP.
Taken together, the 3 last regression sets (Figure 2C-2E) seem to add a new dimension. Unexpectedly, degree of shortening for TROP eyes proved higher for the tail of more mature gestational ages at delivery (Figure 2C). An even more negative slope was obtained with IUGR on the ordinate [given by a small for gestational age (SGA) standard deviation score [65], again to suggest less retardation, the lower the GA (Figure 2D)]. Finally, with the SGA based standard deviation score for growth on abscissa and week 36 adjusted eye size on ordinate (Figure 2E), the degree of intrauterine growth retardation seemed to be a relatively strong determinant for eye size, by its own right. Likewise, IUGR has proven a factor for extra-ocular neurally derived tissue [66].
In short: as a group, the TROP eyes were significantly less grown than average for preterms of a similar gestational age. The growth retardation seemed to be least marked for those most preterm at delivery. In the present sample, the shortest eyes were found among those with higher degree of IUGR, a state that seemed to prevail among those with higher, not lower, gestational age.
After more than 40 years, we are thus roughly closing the Copenhagen circle regarding developmental arrest in the preterm infant and child. Added to the basic age 10-year cohort-based data [12], we can now discuss an early highly selected ?worst scenario? sample of extremely preterm infants, who all presented advanced ROP on the threshold to retinal ablation therapy. It can therefore be concluded, that the full issue is not only about mere environment (the trauma of prematurity), but prenatal factors as here expressed by IUGR also have a role to play. Most likely, a cascade of tissue transmitters is triggered, including a balance between various growth factors as operative. Indirectly, such early pregnancy factors may further underlie the preliminary retinal end result, as here: progression to threshold ROP.
Ultrasound Biomicroscopy (UBM) and Optic Coherence Tomography (OCT). New Dimensions, True Dimensions?
The two new examining techniques share the feature of a high resolution and a restricted penetration in depth. The anterior segment is the main target of the 50 MHz UMB, whereas OCT can pinpoint lesions and specify structure in the fundus of the eye.
OCT is also used for measuring ?flat? and/or sagittal dimensions, with the yard stick improved from a millimeter to a micrometer scale. A combined example is the routine sagittal recording of the retinal nerve fibre layer thickness over the posterior pole. As ?flat? features there are diameter, area, and shape of the optic disc [67-69]. The techniques thus also have relevance for current analyses of ocular growth in preterm subjects. Will the main scope of this lecture, the macroscopical growth deficits as hitherto demonstrated primarily by ultrasound, be enriched and widened when adding the potentials of the OCT?
As main findings in normal infants and children there is the indication of postnatal reduction of foveal thickness and the microstructural transition from multilayered to a monolayer of foveal cells. A related issue is the programmed apoptosis of the papillomacular bundle on its way from relative neonatal fullness to adult values. Research in preterm children here suggested an overall thinner retinal nerve fibre layer, except for the sector that comprises the papillomacular bundle [70-74]. A mention also of de Silva?s group and their analyses of normal optic disc-to-fovea distance: from newborn to adult age they outlined ?only limited growth at the highly organized area of the posterior pole? [75]. Their estimate was an 11% increase for disc-to-fovea distance, as compared to the roughly 50% increase for disc size and overall axial eye dimensions. So far, results among preterms are not unanimous. School age data has indicated equal size of optic nerve head irrespective of term, but also trends that indicate a relative arrest of axonal and neuronal development in preterms. Some variation can be ascribed to the differences in composition of preterm series. Growth restricted features thus seem more frequent among subjects with a history of advanced ROP.
With regard to neonatal and infancy findings, the translation from funduscopicoff-axis or lateral format on the retina to precise and true metric distance has remained a challenge [75,77]. This is due to the magnification factors that are operative whenever the optics of the eye conveys a picture back from the fundus, for our interpretation. Classically it was from a Kodak fundus photo, but RetCam equipment and digitized photos later took over [72,75-81]. The more recent optical coherence tomography techniques have addressed, but not eliminated the problem of true lateral dimensions. Laser waves are reflected from structures in the fundus and optically refracted by the eye itself, prior to the software modulation by way of apparatus algorithms [67,69]. The output details thus partially depend on the optical parameters of the eye, and equipments have depth indicators and calibration gadgets, for instance to compensate for the effects of high ametropia. Maldonado and coworkers discussed the issue in more detail [82]; they included established normative estimates for neonatal and early childhood eye size, but did not take the full step: of using individual values.
By analogy with the Littman corrections, the concerns underlying the above discussion primarily refer to the atypical size and proportions of the infantile eye, so far from what OCT equipments were first developed for. The eyes are small, and peaked corneas are common in preterms. In particular this holds for myopia of prematurity, as also for many eyes otherwise stigmatized by the preterm delivery. Comparing small preterms and full terms, such relations may distort the metric measures. Real differences can be blurred, or differences labeled ?significant? may relate partly to methodical errors. This author feels unable to make the issue more transparent, but would not leave it uncommented. Clearly, the deviant size parameters of growth restricted eyes are worth further studies.
Summary
The author reviews personal experience from ultrasound oculometry studies performed over 46 years. Eye dimensions and the interplay between optical parameters are discussed, and new dimensions have been added to ophthalmic knowledge. With follow-up data supplementing the cohort-based thesis study performed 1969-72 it became clear that preterm survivors - here born around 1960 and of a birth weight under 2000g -apparently never catch up with term children. This held for body height and for eye size. In a large series of apparently normal eyes of 10-year-old preterms axial lengths thus were significantly shorter, and corneas more peaked than in full term controls. The size difference between the two main parameters paralleled that of girls to boys? eyes. The subsequent follow-up at age 18-19 years confirmed the restrained growth of the preterms as a permanent feature.
A general trend of thicker lenses and more shallow anterior chambers in preterms proved even stronger in the sub-group of myopia of prematurity. This sets focus on untoward reactions also in the anterior eye segment as responses to the untimely transfer to extra uterine conditions. Due to the potentially blinding retinopathy of prematurity, till then it was usually the posterior segment that gained attention. However, an early vulnerability of the anterior chamber structures should be no real surprise, considering the early anatomical developing and finishing of the anterior eye segment. This happens within the first 2 years of life, whereas the growth phase of the posterior segment though most marked also that early roughly goes on for another ten years.
Recent reports from other teams have supported that the size deficit of preterms? eyes can be dated back from the neonatal period. Above all, this holds for eyes with ROP, the more advanced stages in particular. In threshold ROP, for instance, it was recently indicated that intrauterine growth retardation perse is a factor underlying not only the restrained growth, but possibly has also a potential of forwarding manifestations of ROP.
In a previous review of eye growth related to preterm delivery[83], we outlined the following:
? Eyes without ROP may appear unaffected, though probably with a slightly depressed eye growth in many. Otherwise we cannot explain the significant findings in the basic 1959-61 cohort. Visual acuity is mainly normal.
? Eyes with reversible and regressed ROP (stages 1-3) are more growth restricted, as expressed by both anterior and posterior segment parameters. Visual acuity is normal, or moderately reduced. In particular, the features hold for myopia of prematurity.
? Eyes with severe ROP (stages 4-5) are usually left with an eye size depending on the early growth that was already attained, when the involutional features of stage 5 ROP became obvious. Blind eyes with classical retrolental fibroplasia appear echo-tight by ultrasound (retinal detachment, fibrosis, membranes) and often of a diameter of 14-15mm.
With a view to teenage years and the general end point of eye growth, we finally dealt with this issue by adding IOL-Master derived data to what was previously achieved by ultrasound. The impressing reproducibility of the new method makes it suited for longitudinal assessment of the decelerating end phase of growth. Usually this was ascribed by Sorsby to age12-13 years, but our data have demonstrated a significant axial elongation also beyond this age. Being subtle, it appears far from the features of the general growth spurt of puberty, and refraction and function often seem to be within normal. The findings, however, suggest a preserved plasticity with regard to eye size. This might be of relevance for not only the entity of adult onset myopia, but also for the occasional adult age progression in established juvenile myopia.
In the long-term context, a final mention further of the fact that eyes more or less ?scarred? by reversible ROP are not just safe for a lifetime. Having kind of lost ?biological reserve?, they may definitely have a propensity for involutional changes and complications, at all subsequent ages [84]. The general recommendation of regular ophthalmic surveillance for preterms at risk thus relates not only to early strabismus, amblyopia, and refraction, but also to the detection of eventual retinal, or other serious complications, and to the measures to be taken.
Disclosure
We have no conflict of interests to declare.
References
- Fich M, Fledelius HC (1993) Intraocular lens prediction and oculometric harmony. With special reference to skew ratios between axial length and corneal curvature radius. Acta Ophthalmol 71: 408-10.
- Olsen T (1992) Sources of error in intraocular lens power calculation. J Cataract Refract Surg 18: 125-129.
- Olsen T (2011) Use of fellow eye data in the calculation of intraocular lens power for the second eye. Ophthalmology 118: 1710-1715.
- Haigis W (2009) IOL calculation using paraxial matrix optics.Ophthalmic Physiol Opt 29: 458-463.
- Haigis W (2012) Influence of axial length on IOL constants. Acta Clin Croat 1: 59-64.
- Zachau-Christiansen B (1972) The influence of prenatal and perinatal factors on development during the first year of life.Copenhagen University thesis. Andersens Forlag, Elsinore.
- Gernet H (1964) Axis Length And Refraction Of The Living Eye In Newborn Infants. Albrecht Von Graefes Arch Ophthalmol 166: 530-536.
- Luyckx J (1966) Measurement of the optic components of the eye of the newborn by ultrasonic echography. 26: 159-170
- Larsen J (1971) The sagittal growth of the eye. IV. Ultrasonic measurement of the axial length of the eye from birth to puberty. Acta Ophthalmol 49: 873-886.
- Blomdahl S (1979) Ultrasonic measurements of the eye in the new born infant. Acta Ophthalmol 57: 1048-1056.
- Grignolo A, Rivara A (1968) Biometry of the human eye from the sixth month of pregnancy to the tenth year of life (measurements of the axial length, retinoscopy refraction, total refraction and lens refraction). In: Vanysek J, ed, Diagnostica Ultrasonica in Ophthalmologia, Universita JE Purkyne Brno: 251-7
- Fledelius HC (1976) Prematurity and the Eye (1976) Copenhagen University thesis. Acta Ophthalmol ,suppl 128.
- Fletcher MC, Brandon S (1955) Myopia of prematurity. Am J Ophthalmol 40: 474-481.
- Birge HL (1956) Myopia caused by prematurity. Am J Ophthalmol 41: 292-298.
- Alfano JE (1958) Myopia of prematurity. Am J Ophthalmol 46: 45-49.
- Zacharias L, Chisholm JF, Chapman RB (1962) Visual and ocular damage in retrolental fibroplasia. Am J Ophthalmol 53: 337-345.
- Donders F (1864) On the anomalies of accommodation and refraction of the eye; with a preliminary essay on physiological dioptrics.
- Sorsby A, Leary GA (1969) A longitudinal study of refraction and its components during growth. Spec Rep Ser Med Res Counc 309: 1-41.
- Goldschmidt E, Fledelius HC, Larsen FE (1981) Clinical features in high myopia. A 10-year follow-up of a sample of young adults. Springer 28: 233-244.
- Goldschmidt E, Fledelius HC (2010) Clinical features in high myopia. A Danish cohort study of unselected high myopia cases followed from age 14 to age 60. 88: 1-117.
- Francois J, Goes F (1970) Etude échographique de la myopie. Bull Soc Belge Ophtal 154: 415-430.
- Delmarcelle Y, Francois J, Goes F, Collignon-Brach J, Luyckx-Bacus J, et al. (1976) Biométrieoculaireclinicque (oculometrie). Bull Soc Belge Ophtalmol 172:1-608.
- Fledelius HC, Goldschmidt E (2010) Oculometry findings in high myopia at adult age: Considerations based on oculometric follow-up data over 28 years in a cohort-based Danish high myopia series. Acta Ophthalmol 88: 472-478.
- Laursen AB, Fledelius HC (1979) Variations in lens thickness in relation to biomicroscopic type of human senile cataract. Acta Ophthalmol 57: 1-13.
- Fledelius HC (1988) Refraction and eye size in the elderly. A review based on literature, including own results. Acta Ophthalmol 66: 241-248.
- Bengtsson B, Grødum K (1999) Refractive changes in the elderly. Acta Ophthalmol Scand 77:37-39.
- Kempen JH, Mitchell P, Lee KE, Tielsch JM, Eye Diseases Prevalence Research Group et al. (2004) The prevalence of refractive errors among adults in the Unites States, Western Europe, and Australia. Arch Ophthalmol 122: 495-505.
- Hyman L (2007) Myopic and hyperopic refractive error in adults: an overview. Ophthalmic Epidemiol 14: 192-197.
- Fotedar R, Mitchell P, Burlutsky G, Wang JJ (2008) Relationship of 10-year change in refraction to nuclear cataract and axial length findings from an older population. Ophthalmology 115: 1273-1278.
- Fledelius HC (1995) Myopia of prematurity, clinical patterns. A follow-up of Danish children now aged 3-9 years. Acta Ophthalmol Scand 73: 402-406.
- Fledelius HC (1996) Pre-term delivery and subsequent ocular development. A 7-10 year follow-up of children screened 1982-84 for ROP. 3: Refraction, Myopia of prematurity. Acta Ophthalmol Scand 74: 297-300.
- Fledelius HC (2000) Myopia profile in Copenhagen medical students 1996-98. Refractive stability over a century is suggested. Acta Ophthalmol Scand 78: 501-505.
- Francois J, Goes F (1973) Biometry of myopia. Ophthalmologica 167: 49-65.
- Gernet H (1968) Neureszur Ultraschallbiometrie des Auges. In: Vanysek J, ed, Diagnostica Ultrasonica in Ophthalmologia, Universita JE Purkyne Brno: 217-234.
- Shih YF, Chiang TH, Lin LLK (2009) Lens thickness changes among schoolchildren in Taiwan. Invest Ophthalmol Vis Sci 50: 2637-2644.
- Gernet H (1970) Oculometridatenbei Augengesunden. Berdtsch Ophthal Ges Docum Ophthalmol 27: 42-47.
- Francois J, Goes F (1973) Biomètrie de la myopie. Ophthalmologica, Basel 147: 49-65.
- Fledelius HC (1981) Myopia of prematurity. Changes during adolescence. A longitudinal study including ultrasound oculometry. Doc Ophthalmol Proc Ser 29: 217-23.
- Goldschmidt E (1968) On the etiology of myopa. An epidemiological study (thesis). 11-115.
- Jensen H (1991) Myopia progression in young school children. A prospective study of myopia progression and the effect of a trial with bifocal lenses and beta blocker eye drops. Acta Ophthalmol 1-79.
- (1984) An international classification of retinopathy of prematurity. The Committee for the Classification of Retinopathy of Prematurity. Arch Ophthalmol 102: 1130-1134.
- Fledelius HC, Bangsgaard R, Slidsborg C, laCour M (2015) Refraction and visual acuity in a national Danish cohort of 4-year-old children of extremely preterm delivery. Acta Ophthalmol 93: electronic doi.10.1111/aos12643 ahead of print.
- Kent D, Pennie F, Laws D, White S, Clark D (2000) The influence of retinopathy of prematurity on ocular growth. Eye 14: 23-29.
- Laws DE, Haslett R, Ashby D, O?Brien C, Clark D (1994) Axial length biometry in infants with retinopathy of prematurity. Eye 8: 427-430.
- Cook A, White S, Batterbury M, Clark D (2008) Ocular growth and refractive error development in premature infants with or without retinopathy of prematurity. Invest Ophthalmol Vis Sci 49: 5199-5207.
- O?Brien C, Clark D (1994) Ocular biometry in preterm infants without retinopathy of prematurity. Eye 8: 662-665.
- Fledelius HC (2000) Retinopathy and myopia of prematurity. Br J Ophthalmol 84: 937.
- Fledelius HC, Fledelius C (2012) Eye size in threshold retinopathy of prematurity, based on a Danish preterm infant series: early axial growth, pre- and postnatal aspects. Invest Ophthalmol Vis Sci 53: 4177-4184.
- Fledelius HC (1981) The axial growth of the eye from age 10 to 18 years. Docum Ophthal Proc Ser 29: 211-216.
- Fledelius HC (1982) Ophthalmic changes from age to of 10 to 18 years. A longitudinal study of sequels to low birth weight IV: Ultrasound oculometry of vitreous and axial length. Acta Ophthalmol 60: 403-411.
- Fledelius HC (1982) Inhibited growth and development as permanent features of low birth weight. A longitudinal study of eye size, height, head circumference, interpupillary distance and exophthalmometry, as measured at age 10 and 18 years. Acta Paediatr Scand 71: 645-650.
- Sorsby A, Benjamin B, Sheridan M, Stone J, Leary Ga (1961) Refraction and its components during the growth of the eye from the age of three. Memo Med ResCounc Rep 301: 1-67.
- Zadnik K, Mutti DO, Mitchell GL, Jones LA, Burr D, et al. (2004) Normal eye growth in emmetropic schoolchildren. Optom Vis Sci 81: 819-828.
- Fledelius HC (1995) Myopia of adult onset. Can analyses be based on patient memory? Acta Ophthalmol Scand 73: 394-396.
- Fledelius HC (1995) Adult onset myopia, oculometric features. Acta Ophthalmol Scand73: 397-401.
- Fledelius HC, Christensen AS (1996) Reappraisal of the human ocular growth curve in fetal life, infancy and early childhood. Br J Ophthalmol 80: 918-921.
- Fledelius HC, Christensen AS, Fledelius C (2014) Juvenile eye growth, when completed? An evaluation based on IOL-Master axial length data, cross-sectional and longitudinal. Acta Ophthalmol 92: 259-264.
- Tane S, Kohno J (1984) Ultrasonic biometry of the sagittal growth of the eyes in children. Docum Ophthalmol Proc Ser Junk Publ: 277-293.
- Hoffer KJ, Aramberri J, Haigis W, Norrby S, Olsen T, et al. (2012) The final frontier: pediatric intraocular lens power. Editorial, Am J Ophthalmol 154: 1-2.
- Fledelius HC (1996) Pre-term delivery and subsequent ocular development. A 7-10 year follow-up of children screened 1982-84 for ROP. 1: visual function, slit lamp findings and fundus appearance. Acta Ophthalmol Scand 74: 288-293.
- Fledelius HC (1996) Pre-term delivery and subsequent ocular development. A 7-10 year follow-up of children screened 1982-84 for ROP. 4: oculometric - and other metric considerations. Acta Ophthalmol Scand 74: 301-305.
- Fledelius HC (1990) Eye size of the premature infant around presumed term. In: Sampaolesi R (ed.) Ultrason in Ophthalmol (SIDUO 1988), Docum Ophthal Proc Ser 53: 165-72.
- Fledelius HC (1990) Ocular features other than retinopathy of prematurity in the preterm infant. Acta Ophthalmol 68: 214-217.
- Fledelius HC (1992) Preterm delivery and the growth of the eye. An oculometric study of eye size around term-time. Acta Ophthalmol Suppl 204: 10-15.
- Quinn GE, Johnson L, Abbasi S (1992) Onset of retinopathy of prematurity as related to postnatal and postconceptional age. Br J Ophthalmol 76: 284-288.
- Marsal K, Perrson PH, Larsen T, Lilja H, Selbing A, et al. (1996) Intrauterine growth curves based on ultrasonically estimated foetal weights. Acta Paediatr 85: 843-848.
- Toft PB, Leth H, Ring PB, Lou HC, Henriksen O, et al. (1995) Volumetric analysis of the normal infant brain and in intrauterine growth retardation. Early Hum Dev 43: 15-29.
- Oliveira C, Harizman N, Girkin CA, Xie A, Tello C, et al. (2007) Axial length and optic disc size in normal eyes. Br J Ophthalmol 91: 37-39.
- Samarawickrama C, Huynh SC, Liew G, Burlutsky G, Mitchell PP (2009) Birth weight and optic nerve head parameters. Ophthalmology 116: 1112-1118.
- Tong AY, El-Dairi M, Maldonado RS, Rothman AL, Yuan EL, et al. (2014) Evaluation of optic nerve development in preterm and term infants using handheld spectral-domain optical coherence tomography. Ophthalmology 121: 1818-1826.
- Åkerblom H, Holmström G, Eriksson U, Larsson E (2012) Retinal nerve fibre layer thickness in school-aged prematurely-born children compared to children born at term. Br J Ophthalmol 96: 956-960.
- Åkerblom H, Larsson E, Eriksson U, Holmström G (2012) Central macular thickness is correlated with gestational age at birth in prematurely born children. Br J Ophthalmol 95: 799-803.?
- Wang J, Spencer R, Leffler JN, Birch EE (2012) Characteristics of peripapillary retinal nerve fiber layer in preterm children. Am J Ophthalmol 153: 850-855.
- Wu WC, Lin RI, Shih CP, Wang NK, Chen YP, et al. (2012) Visual acuity, optical components, and macular abnormalities in patients with a history of retinopathy of prematurity. Ophthalmology 119: 1907-1916.
- Dubis AM, Subramaniam CD, Godara P, Carroll J, Costakos DM (2013) Subclinical macular findings in infants screened for retinopathy of prematurity with Spectral-domain optical coherence tomography. Ophthalmology 120: 1665-1671.
- De Silva DJ, Cocker KD, Lau G, Clay ST, Fielder A, et al. (2006) Optic disk size and optic disk-to-fovea distance in preterm and full-term infants. Invest Ophthalmol Vis Sci 47: 4683-4686.
- Littman (1982) Determination of the real size of an object on the fundus of the living eye. Klin Monbl Augenheilkd 180: 286-289.
- Fledelius HC (2008) Optic disc size: are methodological factors taken into account? Acta Ophthalmol 86: 813-814.
- Barr DB, Weir CR, Purdie AT (1999) An appraisal of the disc-macula distance to disc diameter ratio in the assessment of optic disc size. Ophthalmic Physiol Opt 19: 365-375.
- Hellström A, Engström E, Hard A-L, Albertsson-Wikland K, Carlsson B, et al. Postnatal serum insulin-like growth factor I deficiency is associated with retinopathy of prematurity and other complications of premature birth. Pediatrics 112: 1016-1020.
- Hellström A, Smith LEH, Dammann O (2013) Retinopathy of prematurity. Lancet 382: 1445-1453.
- Ley D, Marsal K, Dahlgren J, Hellström A (2004) Abnormal retinal optic nerve morphology in young adults after intrauterine growth restriction. Pediatr Res 56: 139-143.
- Maldonado RS, Izatt JA, Sarin N, Wallace DK, Freedman S, et al. (2010) Optimizing hand-held spectral domain optical coherence tomography imaging for neonates, infants and children. Invest Ophthalmol Vis Sci 51: 2678-2685.
- Fledelius HC (1995) Eye size, refraction, and ocular morbidity. An ultrasound oculometry review. In Proceedings of the 14th SIDUO Congress 1992, Tokyo, Japan. Docum Ophthalmol Proc Ser 58, Kluwer Academic Publishers: 39-47.
- Fledelius HC, Jensen H (2011) Late subsequent ocular morbidity in retinopathy of prematurity patients, with emphasis on visual loss caused by insidious ?involutive? pathology: an observational series. Acta Ophthalmol 89: 316-323.